CALCIUM PNV- doconexent, ethyl icosapentate, tricalcium phosphate, ferrous fumarate, ascorbic acid, pyridoxine hydrochloride, .alpha.-tocopherol, d- and folic acid tablet, coated
Virtus Pharmaceuticals LLC
----------
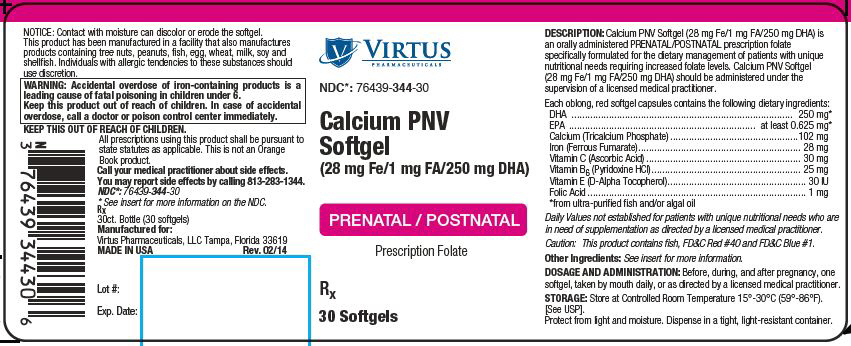
Calcium PNV Softgel (28 mg Fe/1 mg FA/250 mg DHA)
STATEMENT OF IDENTITY: Calcium PNV Softgel (28 mg Fe/1 mg FA/250 mg DHA) is an orally administered prescription dietary supplement and should be administered under the supervision of a licensed medical practitioner.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
Ingestion of more than 3 grams of omega-3 fatty acids per day has been shown to have potential antithrombotic effects, including an increased bleeding time and INR. Administration of omega-3 fatty acids should be avoided in patients on anticoagulants and in those known to have an inherited or acquired bleeding diathesis.
DOSAGE AND ADMINISTRATION: Before, during, and after pregnancy, one softgel, taken by mouth daily, or as directed by a licensed medical practitioner.
Each oblong, red softgel contains the following dietary ingredients:
|
|
| DHA | 250 mg* |
| EPA | at least 0.625 mg* |
| Calcium (Tricalcium Phosphate) | 102 mg |
| Iron (Ferrous Fumarate) | 28 mg |
| Vitamin C (Ascorbic Acid) | 30 mg |
| Vitamin B6 (Pyridoxine HCl) | 25 mg |
| Vitamin E (D-Alpha Tocopherol) | 30 IU |
| Folic Acid | 1 mg |
Other Ingredients: Rice Bran Oil, Gelatin, Glycerin, Water, Beeswax, Lecithin from Sunflower, FD&C Red #40, Titanium Dioxide, FD&C Blue #1 and other ancillary ingredients as needed to ensure product stability.
How Supplied: Calcium PNV Softgel (28 mg Fe/1 mg FA/250 mg DHA) is supplied as oblong, red soft gelatin capsules imprinted with "V344", dispensed in bottles of 30 softgel capsules.
PRECAUTIONS/WARNING: Folate alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folate in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission may occur while neurological manifestations progress.
Daily ingestion of more than 3 grams per day of omega-3 fatty acids (ALA, EPA, and DHA) may have potential antithrombotic activities, or effects, and may increase bleeding times. Administration of omega-3 fatty acids, including DHA, should be avoided in patients with inherited or acquired bleeding diathesis, including those taking anticoagulants. Exercise caution to ensure that the prescribed dosage of DHA does not exceed 1 gram (1000 mg) per day.
| CALCIUM PNV
doconexent, ethyl icosapentate, tricalcium phosphate, ferrous fumarate, ascorbic acid, pyridoxine hydrochloride, .alpha.-tocopherol, d- and folic acid tablet, coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| scoring | 1 | |
| shape | ||
| size (solid drugs) | 24 mm | |
| imprint | ||
| Labeler - Virtus Pharmaceuticals LLC (969483143) |