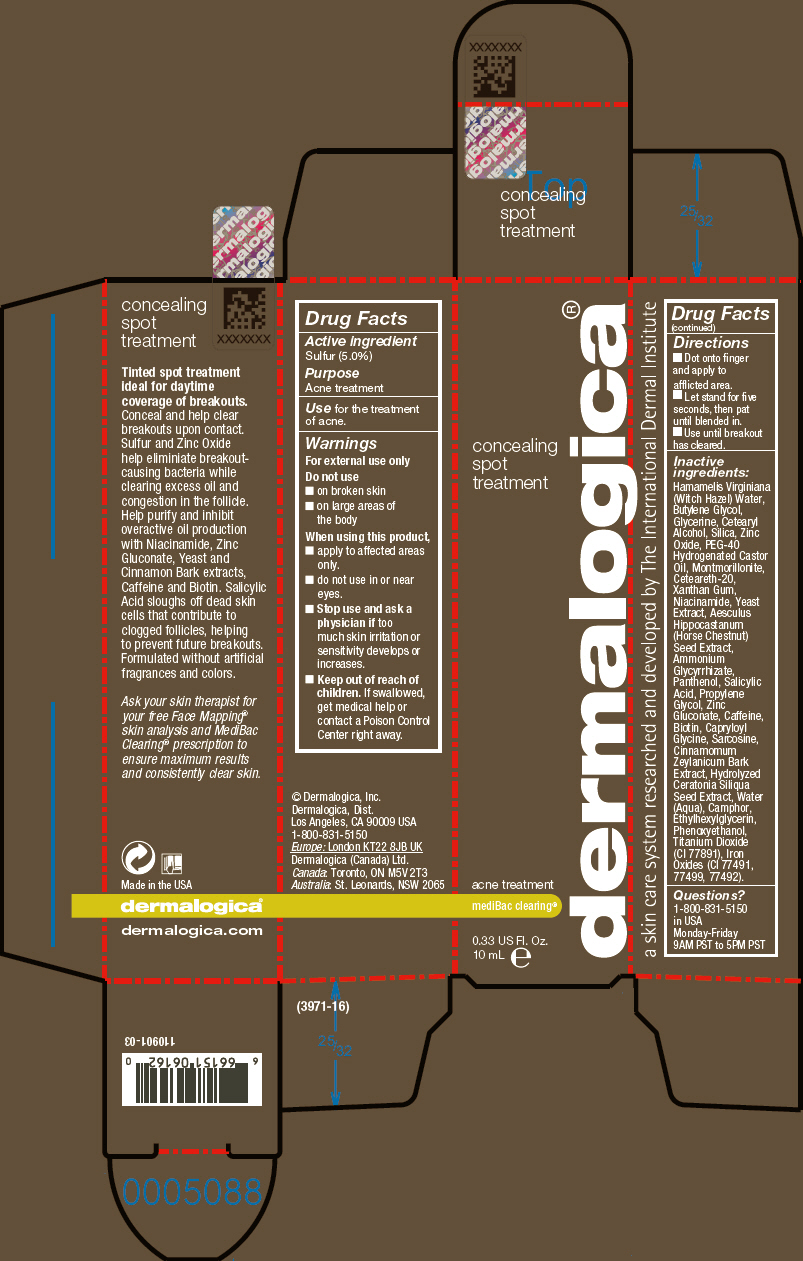
CONCEALING SPOT TREATMENT- sulfur lotion
Dermalogica, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Sulfur (5.0%)
Use
for the treatment of acne.
Warnings
For external use only
Do not use
- on broken skin
- on large areas of the body
When using this product,
- apply to affected areas only.
- do not use in or near eyes.
-
Stop use and ask a physician if too much skin irritation or sensitivity develops or increases.
-
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Dot onto finger and apply to afflicted area.
- Let stand for five seconds, then pat until blended in.
- Use until breakout has cleared.
Inactive ingredients
Hamamelis Virginiana (Witch Hazel) Water, Butylene Glycol, Glycerine, Cetearyl Alcohol, Silica, Zinc Oxide, PEG-40 Hydrogenated Castor Oil, Montmorillonite, Ceteareth-20, Xanthan Gum, Niacinamide, Yeast Extract, Aesculus Hippocastanum (Horse Chestnut) Seed Extract, Ammonium Glycyrrhizate, Panthenol, Salicylic Acid, Propylene Glycol, Zinc Gluconate, Caffeine, Biotin, Capryloyl Glycine, Sarcosine, Cinnamomum Zeylanicum Bark Extract, Hydrolyzed Ceratonia Siliqua Seed Extract, Water (Aqua), Camphor, Ethylhexylglycerin, Phenoxyethanol, Titanium Dioxide (CI 77891), Iron Oxides (CI 77491, 77499, 77492).
Questions?
1-800-831-5150 in USA Monday-Friday 9AM PST to 5PM PST
PRINCIPAL DISPLAY PANEL - 10 mL Tube Carton
concealing
spot
treatment
acne treatment
mediBac clearing®
0.33 US Fl. Oz.
10 mL e
dermalogica®
Dermalogica, Inc.