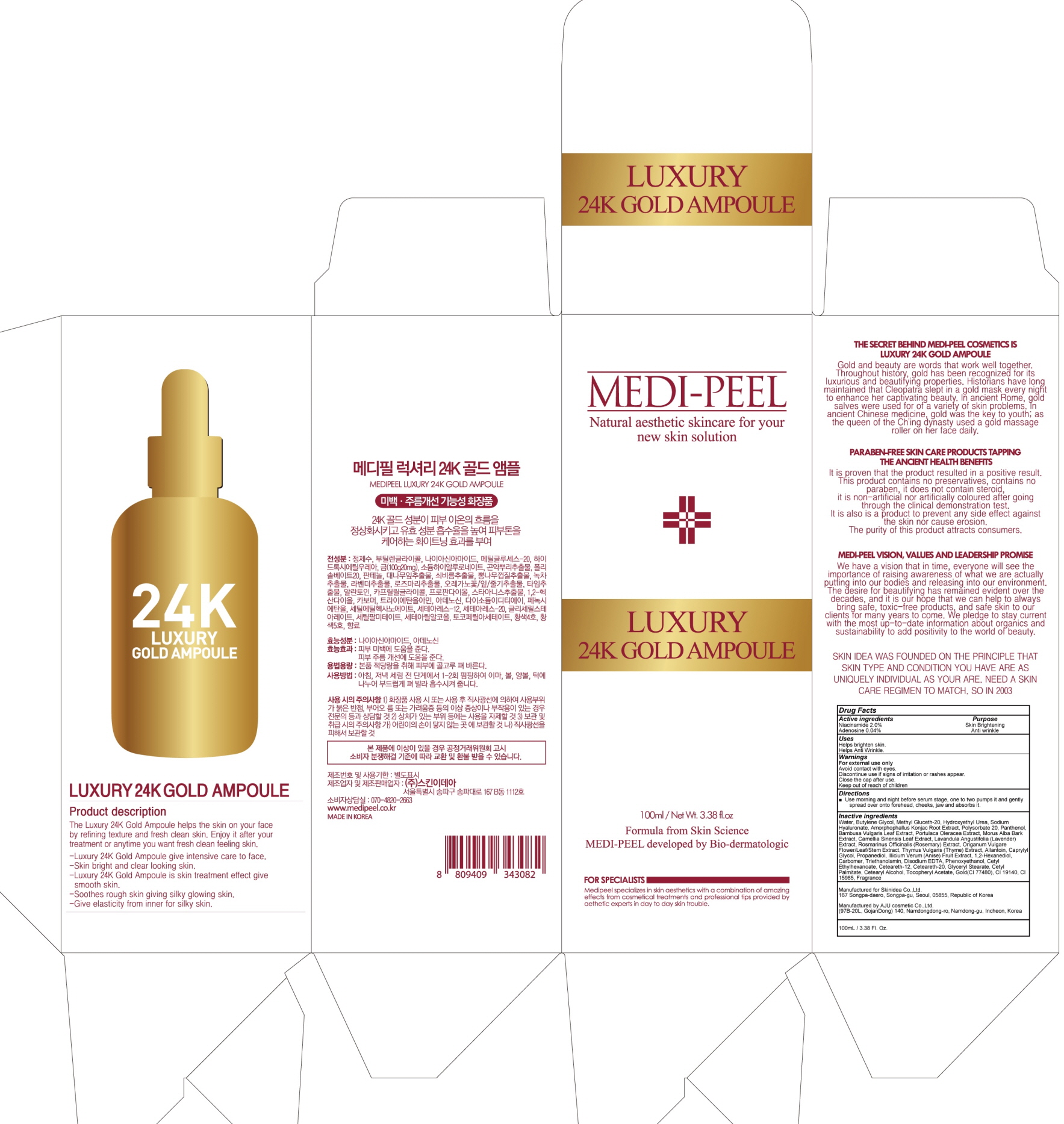
MEDI PEEL LUXURY 24K GOLD AMPOULE- niacinamide, adenosine liquid
Skinidea Co.,Ltd.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
INACTIVE INGREDIENT
Inactive ingredients:
Water, Butylene Glycol, Methyl Gluceth-20, Hydroxyethyl Urea, Sodium Hyaluronate, Amorphophallus Konjac Root Extract, Polysorbate 20, Panthenol, Bambusa Vulgaris Leaf Extract, Portulaca Oleracea Extract, Morus Alba Bark Extract, Camellia Sinensis Leaf Extract, Lavandula Angustifolia (Lavender) Extract, Rosmarinus Officinalis (Rosemary) Extract, Origanum Vulgare Flower/Leaf/Stem Extract, Thymus Vulgaris (Thyme) Extract, Allantoin, Caprylyl Glycol, Propanediol, Illicium Verum (Anise) Fruit Extract, 1,2-Hexanediol, Carbomer, Triethanolamin, Disodium EDTA, Phenoxyethanol, Cetyl Ethylhexanoate, Ceteareth-12, Ceteareth-20, Glyceryl Stearate, Cetyl Palmitate, Cetearyl Alcohol, Tocopheryl Acetate, Gold(CI 77480), CI 19140, CI 15985, Fragrance
WARNINGS
Warnings:
For external use only.
Avoid contact with eyes.
Discontinue use if signs of irritation or rashes appear.
Close the cap after use.
Keep out of reach of children.
| MEDI PEEL LUXURY 24K GOLD AMPOULE
niacinamide, adenosine liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Skinidea Co.,Ltd. (690416325) |
| Registrant - Skinidea Co.,Ltd. (690416325) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Skinidea Co.,Ltd. | 690416325 | manufacture(72220-030) | |