FOSCAVIR UK- foscarnet sodium injection, solution
Clinigen Healthcare Ltd.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
CLINIGEN
Pitcairn House | First Avenue
Burton-on-Trent | DE142WW
United Kingdom
URGENT – FOSCARNET UPDATE
September 2011
Dear Healthcare Professional,
Due to the current critical shortage of foscarnet, Clinigen Healthcare Ltd. is coordinating with the FDA to increase the availability of foscarnet product. FDA approved Foscavir® Injection is no longer available on the United States (US) market, and the generic product foscarnet, manufactured by Hospira Inc., is in critical shortage.
In conjunction with the FDA, Clinigen Healthcare Ltd. has initiated temporary importation into the US market of an international (UK) foscarnet 24 mg/ml product, Foscavir® 24 mg/ml Solution for Infusion to help alleviate foscarnet shortages.
Foscavir® 24 mg/ml Solution for Infusion (UK) contains the same active ingredient, foscarnet sodium1, in the same concentration as the US registered Foscavir®, and is a clinically acceptable substitute to the out of stock US marketed generic Foscavir® product in the United States.
Foscavir® 24 mg/ml Solution for Infusion (UK) is manufactured in FDA inspected facilities, Fresenius Kabi GmBH in Austria. These facilities are currently in compliance with FDA manufacturing standards.
At this time, no other entity except Clinigen Healthcare Ltd. is authorized by the FDA to import or distribute Foscavir® 24 mg/ml Solution for Infusion (UK). With FDA agreement, Clinigen Healthcare Ltd. have appointed Hospira Inc. as their distributor of this product in the US. Any sales of Foscavir® 24 mg/ml Solution for Infusion (UK) bottles from any entity other than Clinigen Healthcare Ltd. or their distributor, Hospira Inc. will be considered a violation of the Federal Food, Drug and Cosmetic Act and will be subject to enforcement by FDA.
Effective immediately, Clinigen Healthcare Ltd. will offer the following product presentations via Hospira Inc.:
| Foscavir® 24 mg/ml Solution for Infusion
(24 mg/ml foscarnet trisodium hexahydrate) | UK Authorisation number: PL 31644/0001 |
| 250 ml bottle supplied in carton. | Overwrapped pack of 10 bottles. |
This product is not the Hospira Inc. approved generic product, foscarnet. Clinigen Healthcare Ltd.'s Foscavir® 24 mg Solution for Infusion (UK) is similar to Foscavir® Injection (US).
It is important to note that there is a difference in the formatting and content of the labeling between the US marketed foscarnet products and the UK Foscavir® 24 mg/ml Solution for Infusion. Due to national requirements, the Foscavir® 24 mg/ml Solution for Infusion (UK) product information sheet contains a short patient information leaflet, not full prescribing information. A copy of this patient information leaflet can be found attached to this communication.
There is no significant difference between US and UK Foscavir®.
- The barcode used for Foscavir® 24 mg/ml Solution for Infusion (UK) is an international pharmaceutical manufacturing code and may not be appropriately recognized by scanning systems used in the United States. Institutions should confirm that barcode systems do not provide incorrect information when the product is scanned.
- Alternative procedures should be followed to assure that the correct drug product is being prepared and administered to individual patients.
- Refer to the Foscavir® US package insert for full prescribing information. (A copy of the US package insert can be found attached to this communication).
- For questions regarding Foscavir® in the United States, please contact the Hospira customer services team at 1 877 946 7747(Monday to Friday 7AM to 6PM CST).
The product comparison table on page 4 also highlights the differences between US and UK Foscavir®.
Customers can order directly from Hospira Inc. by contacting Customer Services on:
- Tel: 1 877 946 7747
- Fax: 1 262 577 6917
Foscavir® 24 mg/ml Solution for Infusion (UK) is not returnable and not for resale.
Hospira Inc., on behalf of Clinigen Healthcare Ltd. will be making reasonable attempts to fill your orders. They will be closely monitoring the distribution of Foscavir® 24 mg/ml Solution for Infusion (UK) to help manage the supply.
If you have additional questions, please contact Customer Service at 1 877 946 7747, Monday – Friday 7AM to 6PM. This communication and updated product information is available on the Clinigen Healthcare Ltd. website (www.clinigen.co.uk), Hospira Inc. website (www.Hospira.com) as well as on the FDA Drug Shortage web site http://www.fda.gov/Drugs/DrugSafety/DrugShortages/default.htm.
To report adverse events among patients administered, please call 011 44 1283 494 340 (07:00-13:00 (ET)), fax 011 44 1283 494 341, or email usf@clinigen.co.uk. Adverse events that may be related to the use of this product may also be reported to FDA's MedWatch Adverse Event Reporting Program either online, by regular mail or by fax:
- Online: www.fda.gov/medwatch/report.htm
- Regular Mail: use postage-paid FDA form 3500 available at:
www.fda.gov/MedWatch/getforms.htm
Mail to MedWatch, FDA, 5600 Fishers Lane, Rockville, MD 20852-9787 - Fax: 1-800-FDA-0178
We urge you to contact the Hospira Customer Services department at 1 877 946 7747 Monday – Friday 7AM to 6PM) outside of these working hours if you have any questions about the information contained in this letter or the safe and effective use of Foscavir® 24 mg/ml Solution for Infusion (UK).
Sincerely,
Jason Shelley, Regulatory Manager, Clinigen Healthcare Ltd.
- 1
- known in the UK as foscarnet trisodium hexahydrate
CARTON LABEL – PRINCIPAL DISPLAY PANEL
Foscavir® 24 mg/ml
Solution for Infusion
Foscarnet trisodium
hexahydrate
For intravenous use
250 ml
Each 250 ml bottle contains 6g of Foscarnet trisodium hexahydrate.
Also contains Water for Injections and hydrochloric acid.
Dilute before injection into a peripheral vein.
Read the package leaflet before use.
Do not store above 30°C. Do not refrigerate. If refrigerated or exposed to temperatures below freezing point precipitation may occur. By keeping the bottle at room temperature with repeated shaking, the precipitate can be brought into solution again.
Clinigen Healthcare Ltd., Pitcairn House, First Avenue, Burton-on-Trent, Staffordshire,
DE14 2WW, UK
PL 31644/0001
Foscavir is a trademark of Clinigen Healthcare Ltd.
POM
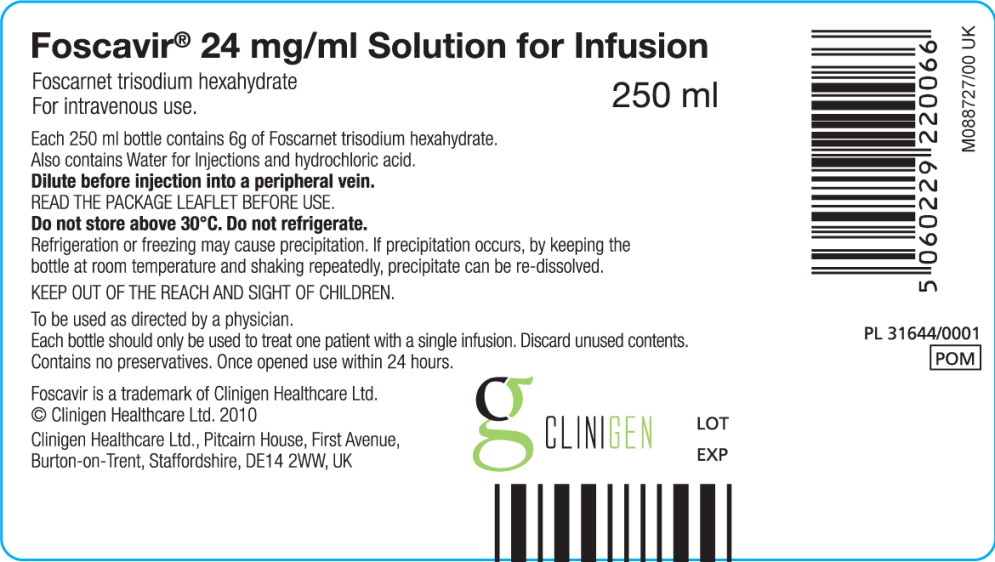
BOTTLE LABEL – PRINCIPAL DISPLAY PANEL
Foscavir® 24 mg/ml Solution for Infusion
Foscarnet trisodium hexahydrate
For intravenous use. 250 ml
Each 250 ml bottle contains 6g of Foscarnet trisodium hexahydrate.
Also contains Water for Injections and hydrochloric acid.
Dilute before injection into a peripheral vein.
READ THE PACKAGE LEAFLET BEFORE USE.
Do not store above 30°C. Do not refrigerate.
Refrigeration or freezing may cause precipitation. If precipitation occurs, by keeping the bottle at room temperature and shaking repeatedly, precipitate can be re-dissolved.
KEEP OUT OF THE REACH AND SIGHT OF CHILDREN.
To be used as directed by a physician.
Each bottle should only be used to treat one patient with a single infusion. Discard unused contents.
Contains no preservatives. Once opened use within 24 hours.
Foscavir is a trademark of Clinigen Healthcare Ltd.
© Clinigen Healthcare Ltd. 2010
Clinigen Healthcare Ltd., Pitcairn House, First Avenue,
Burton-on-Trent, Staffordshire, DE14 2WW, UK
| FOSCAVIR UK
foscarnet sodium injection, solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Clinigen Healthcare Ltd. (856162487) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Fresenius Kabi Austria GmbH | 300206604 | MANUFACTURE(76310-001), ANALYSIS(76310-001), PACK(76310-001) | |