DR. SHEFFIELD BARRIER CREAM- barrier cream cream
Sheffield Pharmaceuticals, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
Multipurpose moisture barrier that aids in protecting irritated skin conditions in the perineum, buttocks, lower abdomen and inner thigh due to moisture, occlusion, chafing or continued contact with urine or feces.
Helps relieve the local itching, discomfort, pain, burning and irritation associated with anorectal disorders and hemorroids.May provide a cooling sensation.
Warnings
Stop use and consult a doctor
•in case of bleeding
•if conditions worsenor deos not improve within 7 days
<Do not exceed recommended daily dosage unless directed by a doctorDo not put prodcut into rectum by using fingers or any mechanical device or applicatorAllergy Alert:Certain persons can develop allergic reactions to this product. If the symptoms develop or increase,discontinue use and consult a doctor. >
Directions
<Adults: When practical ,cleanse the affected areawith a mild soap and warm water and rinse thorughly. Gently dry by pattingor blotting with toilet tissue or a soft cloth beforeapplication of this product.Children under 12 years of age: consult a doctor. Apply extrenally to the affectedarea u pto 6 times a day.>
Inactive ingredients
Calamine, Codliver Oil, Disodium EDTA, Lanolin, Methylparaben, Microcrystalline Wax, Mineral Oil, Petrolatum, Propylparaben, Sodium Borate, Sorbitan Sequioleate, Talc, Water (Purified)
Principal Display Panel - Tube Label

NDC 11527-213-22
Dr. Sheffield's
Made in the USA
Dual action Barrier Cream with pain relief
0.45% Menthol -External Analgesic
20% Zinc Oxide -Astringent
• Protect and soothes irritated or chafed skin associated with incontinence
• Temporary relief of pain & itching
NET WT. 4 oz. (113g)

Principal Display Panel - Packet

NDC # 11527-213-75
Dr. Sheffield's Made in the USA
Dual Action Barrier Cream with Pain Relief
0.45% Menthol - External Analgesic
20% Zinc Oxide -Astrigent
- Soothes and protects irritaed or chafed skin associated with incontinence
- Temporarily relief of pain & itching
NET WT 4g( 1/7oz)
Principal Display Panel - Carton
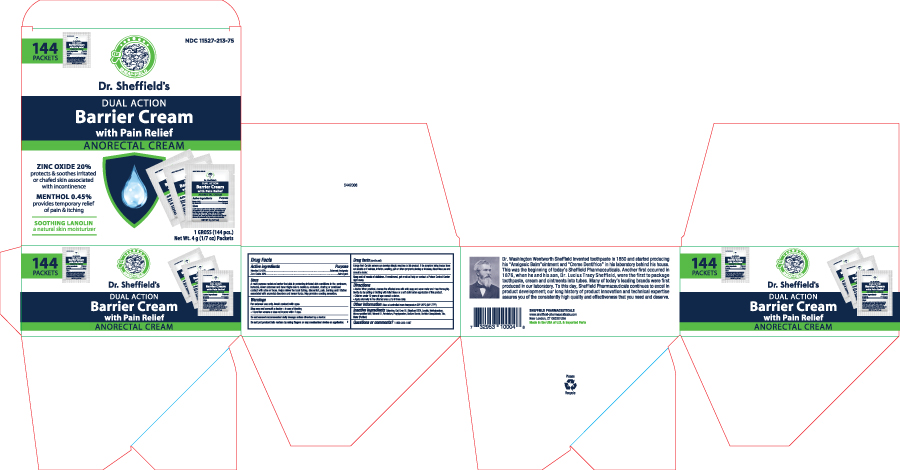
NDC# 11527-213-75
Dr. Sheffield's Made in the USA
Dual Action Barrier Cream
0.45% Menthol
20% Zinc Oxide
- Soothes and protects irritated or chafed skin associated with incontinence
- Provided temporary relief of pain & itching
| DR. SHEFFIELD BARRIER CREAM
barrier cream cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Sheffield Pharmaceuticals, LLC (151177797) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sheffield Pharmaceuticals, LLC | 151177797 | MANUFACTURE(11527-213) | |