PRENA1- ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, 5-methyltetrahydrofolic acid, cyanocobalamin, biotin, calcium pantothenate, ferrous fumarate, zinc oxide and doconexent capsule, gelatin coated
BocaGreenMD Inc.
----------
Prena1
with Quatrefolic®
DESCRIPTION
Prena1 is a prescription prenatal/postnatal dietary supplement containing vitamins, minerals and vegetarian DHA within a purple, opaque softgel.
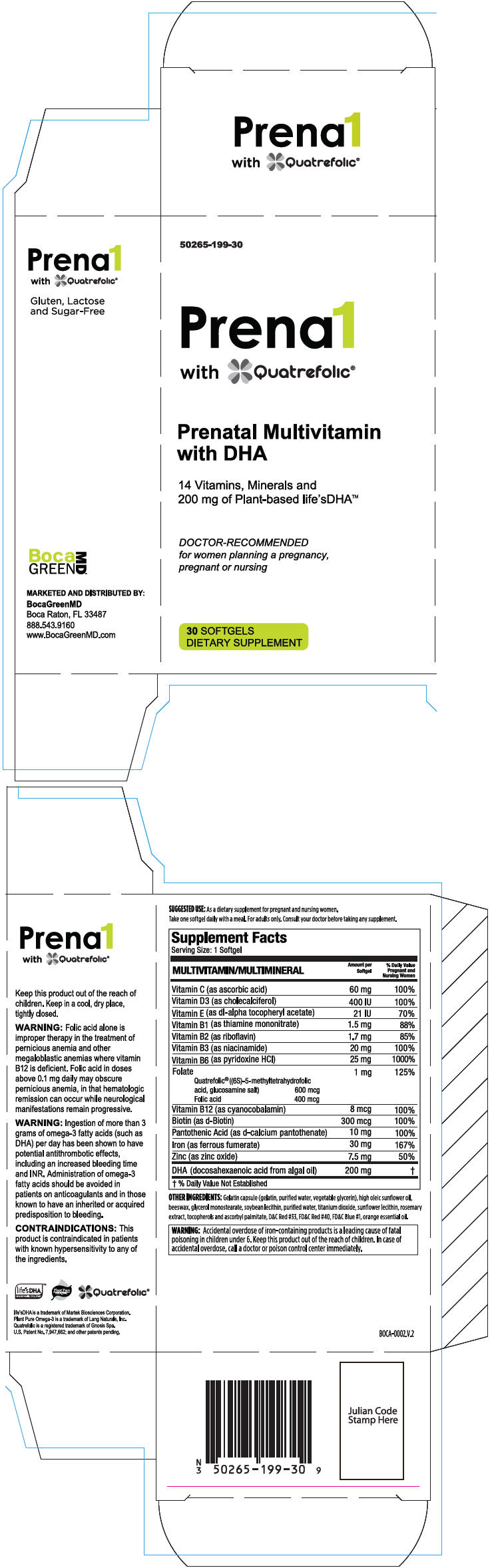
Supplement Facts
Serving Size: 1 Softgel
| MULTIVITAMIN/MULTIMINERAL | Amount per Softgel | % Daily Value Pregnant and Nursing Women | |
|---|---|---|---|
|
|||
| Vitamin C (as ascorbic acid) | 60 mg | 100% | |
| Vitamin D3 (as cholecalciferol) | 400 IU | 100% | |
| Vitamin E (as dl-alpha tocopheryl acetate) | 21 IU | 70% | |
| Vitamin B1 (as thiamine mononitrate) | 1.5 mg | 88% | |
| Vitamin B2 (as riboflavin) | 1.7 mg | 85% | |
| Vitamin B3 (as niacinamide) | 20 mg | 100% | |
| Vitamin B6 (as pyridoxine HCl) | 25 mg | 1000% | |
| Folate | 1 mg | 125% | |
| Quatrefolic® ((6S)-5-methyltetrahydrofolic | |||
| acid, glucosamine salt) | 600 mcg | ||
| Folic acid | 400 mcg | ||
| Vitamin B12 (as cyanocobalamin) | 8 mcg | 100% | |
| Biotin (as d-Biotin) | 300 mcg | 100% | |
| Pantothenic Acid (as d-calcium pantothenate) | 10 mg | 100% | |
| Iron (as ferrous fumerate) | 30 mg | 167% | |
| Zinc (as zinc oxide) | 7.5 mg | 50% | |
| DHA (docosahexaenoic acid from algal oil) | 200 mg | * | |
OTHER INGREDIENTS
Gelatin capsule (gelatin, purified water, vegetable glycerin), high oleic sunflower oil, beeswax, glycerol monostearate, soybean lecithin, purified water, titanium dioxide, sunflower lecithin, rosemary extract, tocopherols and ascorbyl palmitate, D&C Red #33, FD&C Red #40, FD&C Blue #1, orange essential oil.
INDICATIONS
Prena1 is a dietary supplement with DHA indicated for use in improving the nutritional status of women throughout pregnancy and in the postnatal period for both lactating and non-lactating mothers. Prena1 can also be beneficial in improving the nutritional status of women prior to conception.
CONTRAINDICATIONS
This product is contraindicated in patients with known hypersensitivity to any of the ingredients.
| WARNING
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of the reach of children. In case of accidental overdose, call a doctor or poison control center immediately. |
WARNING
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia, in that hematologic remission can occur while neurological manifestations remain progressive.
WARNING
Ingestion of more than 3 grams of omega-3 fatty acids (such as DHA) per day has been shown to have potential antithrombotic effects, including an increased bleeding time and INR. Administration of omega-3 fatty acids should be avoided in patients on anticoagulants and in those known to have an inherited or acquired predisposition to bleeding.
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
DOSAGE AND ADMINISTRATION
As a dietary supplement, take one softgel daily with a meal. For adults only. Consult your doctor before taking any supplement.
| PRENA1
ascorbic acid, cholecalciferol, .alpha.-tocopherol acetate, dl-, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, 5-methyltetrahydrofolic acid, cyanocobalamin, biotin, calcium pantothenate, ferrous fumarate, zinc oxide, and doconexent capsule, gelatin coated |
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - BocaGreenMD Inc. (078441811) |