AMILORIDE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE- amiloride hydrochloride and hydrochlorothiazide tablet
Mylan Pharmaceuticals Inc.
----------
DESCRIPTION
Amiloride hydrochloride and hydrochlorothiazide tablets, USP combine the potassium-conserving action of amiloride hydrochloride with the natriuretic action of hydrochlorothiazide.
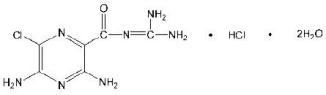
Amiloride hydrochloride is designated chemically as 3,5-diamino-6-chloro-N-(diamino-methylene)pyrazinecarboxamide monohydrochloride, dihydrate. It has the following structural formula:
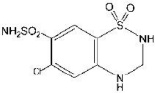
Hydrochlorothiazide is designated chemically as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. It has the following structural formula:
It is a white, or practically white, crystalline powder which is slightly soluble in water, but freely soluble in sodium hydroxide solution.
Each tablet, for oral administration, contains 5 mg of amiloride hydrochloride, USP (calculated on the anhydrous basis) and 50 mg of hydrochlorothiazide, USP. In addition, each tablet contains the following inactive ingredients: croscarmellose sodium, FD&C Yellow No. 6 Aluminum Lake, lactose monohydrate, magnesium stearate, pregelatinized starch (corn), and sodium lauryl sulfate.
CLINICAL PHARMACOLOGY
Amiloride hydrochloride and hydrochlorothiazide tablets provide diuretic and antihypertensive activity (principally due to the hydrochlorothiazide component), while acting through the amiloride component to prevent the excessive potassium loss that may occur in patients receiving a thiazide diuretic. Due to its amiloride component, the urinary excretion of magnesium is less with amiloride and hydrochlorothiazide than with a thiazide or loop diuretic used alone (see PRECAUTIONS). The onset of the diuretic action of this product is within 1 to 2 hours and this action appears to be sustained for approximately 24 hours.
Amiloride Hydrochloride
Amiloride hydrochloride is a potassium-conserving (antikaliuretic) drug that possesses weak (compared with thiazide diuretics) natriuretic, diuretic, and antihypertensive activity. These effects have been partially additive to the effects of thiazide diuretics in some clinical studies. Amiloride hydrochloride has potassium-conserving activity in patients receiving kaliuretic-diuretic agents.
Amiloride hydrochloride is not an aldosterone antagonist and its effects are seen even in the absence of aldosterone.
Amiloride hydrochloride exerts its potassium sparing effect through the inhibition of sodium reabsorption at the distal convoluted tubule, cortical collecting tubule and collecting duct; this decreases the net negative potential of the tubular lumen and reduces both potassium and hydrogen secretion and their subsequent excretion. This mechanism accounts in large part for the potassium sparing action of amiloride.
Amiloride hydrochloride usually begins to act within 2 hours after an oral dose. Its effect on electrolyte excretion reaches a peak between 6 to 10 hours and lasts about 24 hours. Peak plasma levels are obtained in 3 to 4 hours and the plasma half-life varies from 6 to 9 hours. Effects on electrolytes increase with single doses of amiloride hydrochloride up to approximately 15 mg.
Amiloride hydrochloride is not metabolized by the liver but is excreted unchanged by the kidneys. About 50% of a 20 mg dose of amiloride hydrochloride is excreted in the urine and 40% in the stool within 72 hours. Amiloride hydrochloride has little effect on glomerular filtration rate or renal blood flow. Because amiloride hydrochloride is not metabolized by the liver, drug accumulation is not anticipated in patients with hepatic dysfunction, but accumulation can occur if the hepatorenal syndrome develops.
Hydrochlorothiazide
The mechanism of the antihypertensive effect of thiazides is unknown. Thiazides do not usually affect normal blood pressure.
Hydrochlorothiazide is a diuretic and antihypertensive. It affects the distal renal tubular mechanism of electrolyte reabsorption. Hydrochlorothiazide increases excretion of sodium and chloride in approximately equivalent amounts. Natriuresis may be accompanied by some loss of potassium and bicarbonate.
After oral use diuresis begins within 2 hours, peaks in about 4 hours and lasts about 6 to 12 hours.
Hydrochlorothiazide is not metabolized but is eliminated rapidly by the kidney. When plasma levels have been followed for at least 24 hours, the plasma half-life has been observed to vary between 5.6 and 14.8 hours. At least 61% of the oral dose is eliminated unchanged within 24 hours. Hydrochlorothiazide crosses the placental but not the blood-brain barrier and is excreted in breast milk.
INDICATIONS AND USAGE
Amiloride hydrochloride and hydrochlorothiazide tablets are indicated in those patients with hypertension or with congestive heart failure who develop hypokalemia when thiazides or other kaliuretic diuretics are used alone, or in whom maintenance of normal serum potassium levels is considered to be clinically important, e.g., digitalized patients, or patients with significant cardiac arrhythmias.
The use of potassium-conserving agents is often unnecessary in patients receiving diuretics for uncomplicated essential hypertension when such patients have a normal diet.
Amiloride hydrochloride and hydrochlorothiazide tablets may be used alone or as an adjunct to other antihypertensive drugs, such as methyldopa or beta blockers. Since amiloride hydrochloride and hydrochlorothiazide enhances the action of these agents, dosage adjustments may be necessary to avoid an excessive fall in blood pressure and other unwanted side effects.
This fixed combination drug is not indicated for the initial therapy of edema or hypertension except in individuals in whom the development of hypokalemia cannot be risked.
CONTRAINDICATIONS
Hyperkalemia
Amiloride hydrochloride and hydrochlorothiazide tablets should not be used in the presence of elevated serum potassium levels (greater than 5.5 mEq per liter).
Antikaliuretic Therapy or Potassium Supplementation
Amiloride hydrochloride and hydrochlorothiazide should not be given to patients receiving other potassium-conserving agents, such as spironolactone or triamterene. Potassium supplementation in the form of medication, potassium-containing salt substitutes or a potassium-rich diet should not be used with this product except in severe and/or refractory cases of hypokalemia. Such concomitant therapy can be associated with rapid increases in serum potassium levels. If potassium supplementation is used, careful monitoring of the serum potassium level is necessary.
Impaired Renal Function
Anuria, acute or chronic renal insufficiency, and evidence of diabetic nephropathy are contraindications to the use of amiloride hydrochloride and hydrochlorothiazide. Patients with evidence of renal functional impairment (blood urea nitrogen [BUN] levels over 30 mg per 100 mL or serum creatinine levels over 1.5 mg per 100 mL) or diabetes mellitus should not receive the drug without careful, frequent and continuing monitoring of serum electrolytes, creatinine, and BUN levels. Potassium retention associated with the use of an antikaliuretic agent is accentuated in the presence of renal impairment and may result in the rapid development of hyperkalemia.
WARNINGS
Hyperkalemia
Like other potassium-conserving diuretic combinations, amiloride and hydrochlorothiazide may cause hyperkalemia (serum potassium levels greater than 5.5 mEq per liter). In patients without renal impairment or diabetes mellitus, the risk of hyperkalemia with this combination product is about 1% to 2%. This risk is higher in patients with renal impairment or diabetes mellitus (even without recognized diabetic nephropathy). Since hyperkalemia, if uncorrected, is potentially fatal, it is essential to monitor serum potassium levels carefully in any patient receiving amiloride hydrochloride and hydrochlorothiazide, particularly when it is first introduced, at the time of dosage adjustments, and during any illness that could affect renal function.
The risk of hyperkalemia may be increased when potassium-conserving agents, including amiloride hydrochloride and hydrochlorothiazide, are administered concomitantly with an angiotensin-converting enzyme inhibitor, an angiotensin II receptor antagonist, cyclosporine or tacrolimus (see PRECAUTIONS: Drug Interactions). Warning signs or symptoms of hyperkalemia include paresthesias, muscular weakness, fatigue, flaccid paralysis of the extremities, bradycardia, shock, and ECG abnormalities. Monitoring of the serum potassium level is essential because mild hyperkalemia is not usually associated with an abnormal ECG.
When abnormal, the ECG in hyperkalemia is characterized primarily by tall, peaked T waves or elevations from previous tracings. There may also be lowering of the R wave and increased depth of the S wave, widening and even disappearance of the P wave, progressive widening of the QRS complex, prolongation of the PR interval, and ST depression.
Treatment of Hyperkalemia
If hyperkalemia occurs in patients taking amiloride and hydrochlorothiazide, the drug should be discontinued immediately. If the serum potassium level exceeds 6.5 mEq per liter, active measures should be taken to reduce it. Such measures include the intravenous administration of sodium bicarbonate solution or oral or parenteral glucose with a rapid-acting insulin preparation. If needed, a cation exchange resin such as sodium polystyrene sulfonate may be given orally or by enema. Patients with persistent hyperkalemia may require dialysis.
Diabetes Mellitus
In diabetic patients, hyperkalemia has been reported with the use of all potassium-conserving diuretics, including amiloride hydrochloride, even in patients without evidence of diabetic nephropathy. Therefore, amiloride and hydrochlorothiazide should be avoided, if possible, in diabetic patients and, if it is used, serum electrolytes and renal function must be monitored frequently.
Amiloride and hydrochlorothiazide should be discontinued at least 3 days before glucose tolerance testing.
Metabolic or Respiratory Acidosis
Antikaliuretic therapy should be instituted only with caution in severely ill patients in whom respiratory or metabolic acidosis may occur, such as patients with cardiopulmonary disease or poorly controlled diabetes. If amiloride and hydrochlorothiazide is given to these patients, frequent monitoring of acid-base balance is necessary. Shifts in acid-base balance alter the ratio of extracellular/intracellular potassium, and the development of acidosis may be associated with rapid increases in serum potassium levels.
PRECAUTIONS
General
Electrolyte Imbalance and BUN Increases
Determination of serum electrolytes to detect possible electrolyte imbalance should be performed at appropriate intervals.
Patients should be observed for clinical signs of fluid or electrolyte imbalance: i.e., hyponatremia, hypochloremic alkalosis, and hypokalemia. Serum and urine electrolyte determinations are particularly important when the patient is vomiting excessively or receiving parenteral fluids. Warning signs or symptoms of fluid and electrolyte imbalance, irrespective of cause, include dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, confusion, seizures, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal disturbances such as nausea and vomiting.
Hyponatremia and hypochloremia may occur during the use of thiazides and other diuretics. Any chloride deficit during thiazide therapy is generally mild and may be lessened by the amiloride hydrochloride component of this product. Hypochloremia usually does not require specific treatment except under extraordinary circumstances (as in liver disease or renal disease). Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is water restriction, rather than administration of salt, except in rare instances when the hyponatremia is life threatening. In actual salt depletion, appropriate replacement is the therapy of choice.
Hypokalemia may develop during thiazide therapy, especially with brisk diuresis, when severe cirrhosis is present, during concomitant use of corticosteroids or ACTH, or after prolonged therapy. However, this usually is prevented by the amiloride hydrochloride component of this combination drug product.
Interference with adequate oral electrolyte intake will also contribute to hypokalemia. Hypokalemia may cause cardiac arrhythmia and may also sensitize or exaggerate the response of the heart to the toxic effects of digitalis (e.g., increased ventricular irritability).
Thiazides have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia. Amiloride hydrochloride, a component of this combination product, has been shown to decrease the enhanced urinary excretion of magnesium which occurs when a thiazide or loop diuretic is used alone.
Increases in BUN levels have been reported with amiloride hydrochloride and with hydrochlorothiazide. These increases usually have accompanied vigorous fluid elimination, especially when diuretic therapy was used in seriously ill patients, such as those who had hepatic cirrhosis with ascites and metabolic alkalosis, or those with resistant edema. Therefore, when amiloride and hydrochlorothiazide is given to such patients, careful monitoring of serum electrolyte and BUN levels is important. In patients with preexisting severe liver disease, hepatic encephalopathy, manifested by tremors, confusion, and coma, and increased jaundice, have been reported in association with diuretic therapy including amiloride hydrochloride and hydrochlorothiazide.
In patients with renal disease, diuretics may precipitate azotemia. Cumulative effects of the components of amiloride hydrochloride and hydrochlorothiazide may develop in patients with impaired renal function. If renal impairment becomes evident, amiloride and hydrochlorothiazide should be discontinued (see CONTRAINDICATIONS and WARNINGS).
Drug Interactions
In some patients, the administration of a non-steroidal anti-inflammatory agent can reduce the diuretic, natriuretic, and antihypertensive effects of loop, potassium-sparing and thiazide diuretics. Therefore, when amiloride and hydrochlorothiazide and non-steroidal anti-inflammatory agents are used concomitantly, the patient should be observed closely to determine if the desired effect of the diuretic is obtained. Since indomethacin and potassium-sparing diuretics, including this product, may each be associated with increased serum potassium levels, the potential effects on potassium kinetics and renal function should be considered when these agents are administered concurrently.
Amiloride Hydrochloride
When amiloride hydrochloride is administered concomitantly with an angiotensin-converting enzyme inhibitor, an angiotensin II receptor antagonist, cyclosporine or tacrolimus, the risk of hyperkalemia may be increased. Therefore, if concomitant use of these agents is indicated because of demonstrated hypokalemia, they should be used with caution and with frequent monitoring of serum potassium (see WARNINGS).
Hydrochlorothiazide
When given concurrently the following drugs may interact with thiazide diuretics.
Antidiabetic Drugs (Oral Agents and Insulin)
Dosage adjustment of the antidiabetic drug may be required.
Cholestyramine and Colestipol Resins
Absorption of hydrochlorothiazide is impaired in the presence of anionic exchange resins. Single doses of either cholestyramine or colestipol resins bind the hydrochlorothiazide and reduce its absorption from the gastrointestinal tract by up to 85% and 43%, respectively.
Pressor Amines (e.g., Norepinephrine)
Possible decreased response to pressor amines but not sufficient to preclude their use.
Metabolic and Endocrine Effects
In diabetic patients, insulin requirements may be increased, decreased, or unchanged due to the hydrochlorothiazide component. Diabetes mellitus that has been latent may become manifest during administration of thiazide diuretics.
Because calcium excretion is decreased by thiazides, amiloride and hydrochlorothiazide should be discontinued before carrying out tests for parathyroid function. Pathologic changes in the parathyroid glands, with hypercalcemia and hypophosphatemia have been observed in a few patients on prolonged thiazide therapy; however, the common complications of hyperparathyroidism such as renal lithiasis, bone resorption, and peptic ulceration have not been seen.
Hyperuricemia may occur or acute gout may be precipitated in certain patients receiving thiazide therapy.
Other Precautions
In patients receiving thiazides, sensitivity reactions may occur with or without a history of allergy or bronchial asthma. The possibility of exacerbation or activation of systemic lupus erythematosus has been reported with the use of thiazides.
Increases in cholesterol and triglyceride levels may be associated with thiazide diuretic therapy.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate the effects upon fertility, mutagenicity or carcinogenic potential of amiloride hydrochloride and hydrochlorothiazide.
Amiloride Hydrochloride
There was no evidence of a tumorigenic effect when amiloride hydrochloride was administered for 92 weeks to mice at doses up to 10 mg/kg/day (25 times the maximum daily human dose). Amiloride hydrochloride has also been administered for 104 weeks to male and female rats at doses up to 6 and 8 mg/kg/day (15 and 20 times the maximum daily dose for humans, respectively) and showed no evidence of carcinogenicity.
Amiloride hydrochloride was devoid of mutagenic activity in various strains of Salmonella typhimurium with or without a mammalian liver microsomal activation system (Ames test).
Hydrochlorothiazide
Two-year feeding studies in mice and rats conducted under the auspices of the National Toxicology Program (NTP) uncovered no evidence of a carcinogenic potential of hydrochlorothiazide in female mice (at doses of up to approximately 600 mg/kg/day) or in male and female rats (at doses of up to approximately 100 mg/kg/day). The NTP, however, found equivocal evidence for hepatocarcinogenicity in male mice.
Hydrochlorothiazide was not genotoxic in vitro in the Ames mutagenicity assay of Salmonella typhimurium strains TA 98, TA 100, TA 1535, TA 1537, and TA 1538 and in the Chinese Hamster Ovary (CHO) test for chromosomal aberrations, or in vivo in assays using mouse germinal cell chromosomes, Chinese Hamster bone marrow chromosomes, and the Drosophila sex-linked recessive lethal trait gene. Positive test results were obtained only in the in vitro CHO Sister Chromatid Exchange (clastogenicity) and in the Mouse Lymphoma Cell (mutagenicity) assays, using concentrations of hydrochlorothiazide from 43 to 1300 mcg/mL, and in the Aspergillus nidulans non-disjunction assay at an unspecified concentration.
Hydrochlorothiazide had no adverse effects on the fertility of mice and rats of either sex in studies wherein these species were exposed, via their diet, to doses of up to 100 and 4 mg/kg, respectively, prior to conception and throughout gestation.
Pregnancy
Teratogenic Effects
Teratogenicity studies have been performed with combinations of amiloride hydrochloride and hydrochlorothiazide in rabbits and mice at doses up to 25 times the expected maximum daily dose for humans and have revealed no evidence of harm to the fetus. No evidence of impaired fertility in rats was apparent at dosage levels up to 25 times the expected maximum human daily dose. A perinatal and postnatal study in rats showed a reduction in maternal body weight gain during and after gestation at a daily dose of 25 times the expected maximum daily dose for humans. The body weights of alive pups at birth and at weaning were also reduced at this dose level. There are no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human responses, and because of the data listed below with the individual components, this drug should be used during pregnancy only if clearly needed.
Amiloride Hydrochloride
Teratogenicity studies with amiloride hydrochloride in rabbits and mice given 20 and 25 times the maximum human dose, respectively, revealed no evidence of harm to the fetus, although studies showed that the drug crossed the placenta in modest amounts. Reproduction studies in rats at 20 times the expected maximum daily dose for humans showed no evidence of impaired fertility. At approximately 5 or more times the expected maximum daily dose for humans, some toxicity was seen in adult rats and rabbits and a decrease in rat pup growth and survival occurred.
Hydrochlorothiazide
Teratogenic Effects
Studies in which hydrochlorothiazide was orally administered to pregnant mice and rats during their respective periods of major organogenesis at doses up to 3000 mg and 1000 mg hydrochlorothiazide/kg, respectively, provided no evidence of harm to the fetus. There are, however, no adequate and well controlled studies in pregnant women.
Nursing Mothers
Studies in rats have shown that amiloride is excreted in milk in concentrations higher than those found in blood, but it is not known whether amiloride hydrochloride is excreted in human milk. However, thiazides appear in breast milk. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Geriatric Use
Clinical studies of amiloride hydrochloride and hydrochlorothiazide tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they responded differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function (see CONTRAINDICATIONS: Impaired Renal Function).
ADVERSE REACTIONS
Amiloride hydrochloride and hydrochlorothiazide is usually well tolerated and significant clinical adverse effects have been reported infrequently. The risk of hyperkalemia (serum potassium levels greater than 5.5 mEq per liter) with amiloride hydrochloride and hydrochlorothiazide is about 1% to 2% in patients without renal impairment or diabetes mellitus (see WARNINGS). Minor adverse reactions to amiloride hydrochloride have been reported relatively frequently (about 20%) but the relationship of many of the reports to amiloride hydrochloride is uncertain and the overall frequency was similar in hydrochlorothiazide treated groups. Nausea/anorexia, abdominal pain, flatulence, and mild skin rash have been reported and probably are related to amiloride. Other adverse experiences that have been reported with amiloride and hydrochlorothiazide are generally those known to be associated with diuresis, thiazide therapy, or with the underlying disease being treated. Clinical trials have not demonstrated that combining amiloride and hydrochlorothiazide increases the risk of adverse reactions over those seen with the individual components.
The adverse reactions for amiloride and hydrochlorothiazide listed in the following table have been arranged into two groups: (1) incidence greater than 1%; and (2) incidence 1% or less. The incidence for group (1) was determined from clinical studies conducted in the United States (607 patients treated with amiloride and hydrochlorothiazide). The adverse effects listed in group (2) include reports from the same clinical studies and voluntary reports since marketing. The probability of a causal relationship exists between amiloride and hydrochlorothiazide and these adverse reactions, some of which have been reported only rarely.
| Incidence >1% | Incidence ≤ 1% |
|---|---|
|
|
|
Body as a Whole |
|
|
Headache* |
Malaise |
|
Weakness* |
Chest pain |
|
Fatigue/tiredness |
Back pain |
|
Cardiovascular |
|
|
Arrhythmia |
Tachycardia |
|
Digestive |
|
|
Nausea/anorexia* |
Constipation |
|
Diarrhea |
GI bleeding |
|
Gastrointestinal pain |
GI disturbance |
|
Abdominal pain |
Appetite changes |
|
Metabolic |
|
|
Elevated serum |
Gout |
|
potassium levels |
Dehydration |
|
(> 5.5 mEq per liter)† |
Symptomatic hyponatremia‡ |
|
Musculoskeletal |
|
|
Leg ache |
Muscle cramps/spasm |
|
Nervous |
|
|
Dizziness* |
Paresthesia/numbness |
|
Psychiatric |
|
|
None |
Insomnia |
|
Respiratory |
|
|
Dyspnea |
None |
|
Skin |
|
|
Rash* |
Flushing |
|
Pruritus |
Diaphoresis |
|
Special Senses |
|
|
None |
Bad taste |
|
Urogenital |
|
|
None |
Impotence |
Other adverse reactions that have been reported with the individual components and within each category are listed in order of decreasing severity:
Amiloride
Body as a Whole: Painful extremities, neck/shoulder ache, fatigability;
Cardiovascular: Palpitation;
Digestive: Activation of probable preexisting peptic ulcer, abnormal liver function, jaundice, dyspepsia, heartburn;
Hematologic: Aplastic anemia, neutropenia;
Integumentary: Alopecia, itching, dry mouth;
Nervous System/Psychiatric: Encephalopathy, tremors, decreased libido;
Respiratory: Shortness of breath, cough;
Special Senses: Increased intraocular pressure, tinnitus;
Urogenital: Bladder spasms, polyuria, urinary frequency.
Hydrochlorothiazide
Digestive: Pancreatitis, jaundice (intrahepatic cholestatic jaundice), sialadenitis, cramping, gastric irritation;
Hematologic: Aplastic anemia, agranulocytosis, leukopenia, hemolytic anemia, thrombocytopenia;
Hypersensitivity: Anaphylactic reactions, necrotizing angiitis (vasculitis, cutaneous vasculitis), respiratory distress including pneumonitis and pulmonary edema, photosensitivity, fever, urticaria, purpura;
Metabolic: Electrolyte imbalance (see PRECAUTIONS), hyperglycemia, glycosuria, hyperuricemia;
Nervous System/Psychiatric: Restlessness;
Special Senses: Transient blurred vision, xanthopsia;
Urogenital: Interstitial nephritis (see WARNINGS).
Postmarketing Experience
Non-melanoma Skin Cancer
Hydrochlorothiazide is associated with an increased risk of non-melanoma skin cancer. In a study conducted in the Sentinel System, increased risk was predominantly for squamous cell carcinoma (SCC) and in white patients taking large cumulative doses. The increased risk for SCC in the overall population was approximately 1 additional case per 16,000 patients per year, and for white patients taking a cumulative dose of ≥ 50,000 mg the risk increase was approximately 1 additional SCC case for every 6,700 patients per year.
OVERDOSAGE
No data are available in regard to overdosage in humans. The oral LD50 of the combination drug is 189 and 422 mg/kg for female mice and female rats, respectively.
It is not known whether the drug is dialyzable.
No specific information is available on the treatment of overdosage with amiloride and hydrochlorothiazide, and no specific antidote is available. Treatment is symptomatic and supportive. Therapy with amiloride and hydrochlorothiazide should be discontinued and the patient observed closely. Suggested measures include induction of emesis and/or gastric lavage.
Amiloride Hydrochloride
No data are available in regard to overdosage in humans.
The oral LD50 of amiloride hydrochloride (calculated as the base) is 56 mg/kg in mice and 36 to 85 mg/kg in rats, depending on the strain.
The most common signs and symptoms to be expected with overdosage are dehydration and electrolyte imbalance. If hyperkalemia occurs, active measures should be taken to reduce the serum potassium levels.
Hydrochlorothiazide
The oral LD50 of hydrochlorothiazide is greater than 10 g/kg in both mice and rats.
The most common signs and symptoms observed are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias.
DOSAGE AND ADMINISTRATION
Amiloride hydrochloride and hydrochlorothiazide tablets should be administered with food.
The usual starting dosage is 1 tablet a day. The dosage may be increased to 2 tablets a day, if necessary. More than 2 tablets of amiloride hydrochloride and hydrochlorothiazide daily usually are not needed and there is no controlled experience with such doses. Hydrochlorothiazide can be given at doses of 12.5 mg to 50 mg per day when used alone. Patients usually do not require doses of hydrochlorothiazide in excess of 50 mg daily when combined with other antihypertensive agents.
The daily dose is usually given as a single dose but may be given in divided doses. Once an initial diuresis has been achieved, dosage adjustment may be necessary. Maintenance therapy may be on an intermittent basis.
HOW SUPPLIED
Amiloride Hydrochloride and Hydrochlorothiazide Tablets, USP are available containing 5 mg of anhydrous amiloride hydrochloride, USP and 50 mg of hydrochlorothiazide, USP.
The 5 mg/50 mg tablets are light orange, round, scored tablets debossed with M above the score and 577 below the score on one side of the tablet and blank on the other side. They are available as follows:
NDC 0378-0577-01
bottles of 100 tablets
NDC 0378-0577-05
bottles of 500 tablets
Store at 20º to 25ºC (68º to 77ºF). [See USP Controlled Room Temperature.]
Protect from light.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Revised: 4/2020
AHCTZ:R13
PRINCIPAL DISPLAY PANEL - 5 mg/50 mg
NDC 0378-0577-01
Amiloride HCl and
Hydrochlorothiazide
Tablets, USP
5 mg*/
50 mg
Rx only 100 Tablets
Each tablet contains: Amiloride
Hydrochloride, USP 5 mg*
*(Anhydrous equivalent)
Hydrochlorothiazide, USP 50 mg
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Keep this and all medication
out of the reach of children.
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room
Temperature.]
Protect from light.
Usual Adult Dosage: One or two
tablets daily. See accompanying
prescribing information.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Mylan.com
RM0577A5
| AMILORIDE HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
amiloride hydrochloride and hydrochlorothiazide tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Mylan Pharmaceuticals Inc. (059295980) |