Label: THE BLACK PATCH- menthol patch
-
Contains inactivated NDC Code(s)
NDC Code(s): 71948-101-05, 71948-101-10 - Packager: Carbon Innovation, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 22, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient (in each patch)
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Inactive ingredients
- Questions or Comments?
-
SPL UNCLASSIFIED SECTION
HYDROGEL PAIN RELIEF PATCH with MENTHOL
- Soothing relief for painful joints and muscles
- Lasts up to 8 hours
- Just peel and stick
- Contains activated charcoal for smoother skin!
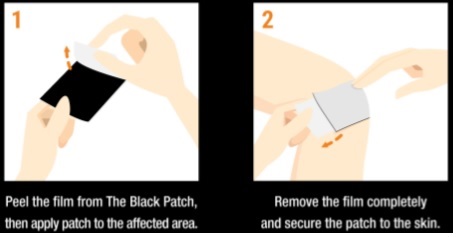
Care Instructions
- The hydrogel must contact the skin for best results. Clean the skin of oil, dirt, lotions, etc. and dry completely.
- It is sometimes helpful to use medical tape around the edges or a tension bandage to hold the patch securely in place.
- The Black Patch is designed to apply easily and remove residue-free. However, due to heat, excessive moisture, or other factors, some hydrogel residue may transfer.
- If residue transfers onto the skin or clothing, simply wash off with warm water or in the laundry.
- The Black Patch is proudly made in America with natural, food grade ingredients. However, it's not edible so keep it out of reach of small children.
Manufactured by
Carbon Innovation
600 Business Park Dr
Lincoln, CA 95648
- Packaging
-
INGREDIENTS AND APPEARANCE
THE BLACK PATCH
menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71948-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 4 g in 100 g Inactive Ingredients Ingredient Name Strength ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) ALCOHOL (UNII: 3K9958V90M) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYVINYL ALCOHOL/POLYVINYL ACETATE COPOLYMER (8:1; 50000 MW) (UNII: 8K8B5SD7ZR) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) TARTARIC ACID (UNII: W4888I119H) WATER (UNII: 059QF0KO0R) Product Characteristics Color black, white (black and white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71948-101-05 5 in 1 PACKAGE 12/30/2017 1 0.348 g in 1 PATCH; Type 0: Not a Combination Product 2 NDC:71948-101-10 10 in 1 BOX 12/30/2017 2 5 in 1 PACKAGE 2 0.348 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 12/30/2017 Labeler - Carbon Innovation, Inc. (080987228) Establishment Name Address ID/FEI Business Operations Carbon Innovation, Inc. 080987228 manufacture(71948-101)


