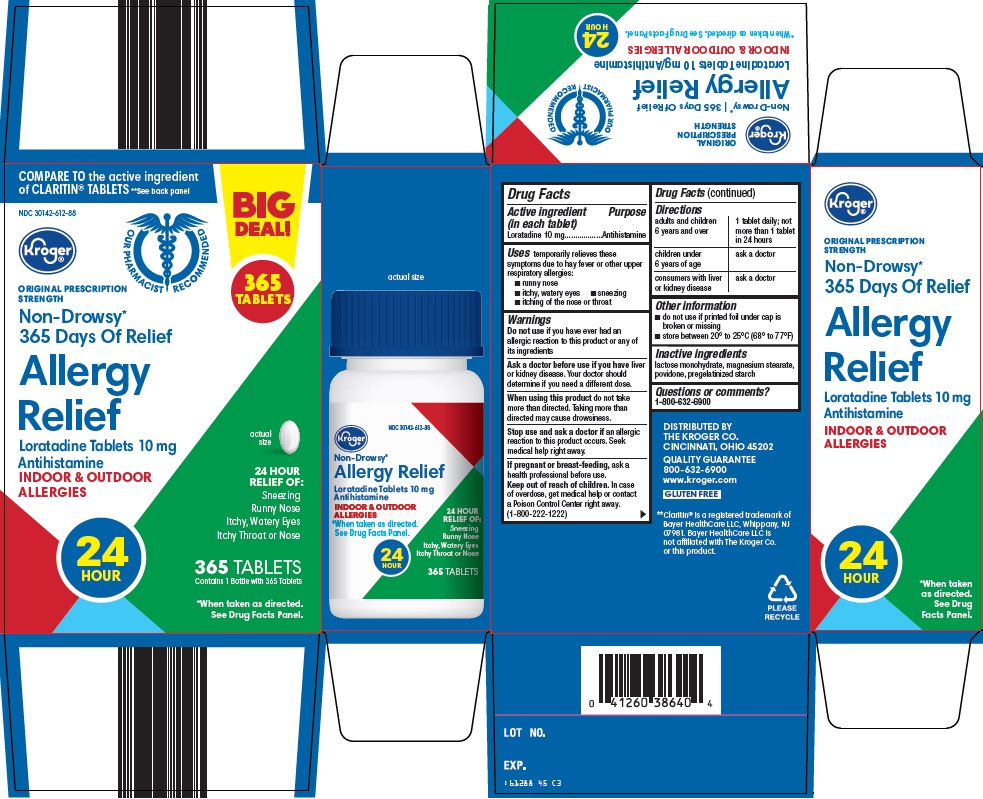
Label: ALLERGY RELIEF- loratadine tablet
-
NDC Code(s):
30142-612-03,
30142-612-46,
30142-612-58,
30142-612-65, view more30142-612-72, 30142-612-76, 30142-612-88, 30142-612-92
- Packager: Kroger Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
COMPARE TO the active ingredient of CLARITIN® TABLETS
See back panel
BIG DEAL! 365 TABLETS
Kroger®
OUR PHARMACIST RECOMMENDED
ORIGINAL PRESCRIPTION STRENGTH
NON-DROWSY*
365 Days Of Relief
Allergy Relief
Loratadine Tablets 10 mg
Antihistamine
INDOOR & OUTDOOR ALLERGIES
actual size
24 HOUR RELIEF OF:
Sneezing
Runny Nose
Itchy, Watery Eyes
Itchy Throat or Nose
24 HOUR
365 TABLETS
Contains 1 Bottle with 365 Tablets
*When taken as directed.
See Drug Facts Panel.
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
loratadine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:30142-612 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) Product Characteristics Color WHITE Score no score Shape OVAL Size 8mm Flavor Imprint Code L612 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:30142-612-46 10 in 1 CARTON 10/08/2005 10/08/2005 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:30142-612-65 1 in 1 CARTON 10/13/2005 03/31/2023 2 30 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:30142-612-72 1 in 1 CARTON 02/04/2006 08/10/2015 3 60 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:30142-612-76 1 in 1 CARTON 08/22/2005 07/17/2019 4 120 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC:30142-612-03 1 in 1 CARTON 03/19/2014 04/30/2023 5 70 in 1 BOTTLE; Type 0: Not a Combination Product 6 NDC:30142-612-58 1 in 1 CARTON 04/05/2014 6 40 in 1 BOTTLE; Type 0: Not a Combination Product 7 NDC:30142-612-92 2 in 1 CARTON 01/16/2018 01/31/2021 7 70 in 1 BOTTLE; Type 0: Not a Combination Product 8 NDC:30142-612-88 1 in 1 CARTON 04/19/2018 8 365 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076301 08/22/2005 Labeler - Kroger Company (006999528)