ARTHRITAZIN PAIN RELIEVING- capsaicin gel
ARTHRITAZIN PAIN RELIEVING EZ- capsaicin gel
Concept Laboratories, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Capsicum Oleoresin 0.0625%
(Containing Capsaicin 0.025%)
Uses
for the temporary relief of minor aches and pains of muscles and joints associated with simple backache arthritis strains sprains
Warnings
For external use only. do not apply to wounds or damaged skin do not bandage tightly do not use with heating pad, wrap, hot water bottle or any heating element.
When using this product do not get into eyes
avoid contact with other mucous membranes
Stop use and ask a doctor if condition worsens if symptoms persist for more than 7 days clear up and occur again within a few days
Keep out of reach of children.
if swallowed, get medical help or contact a Poison Control center right away
if pregnant or nursing, seek the advice of a health professional before using
Directions
Children under 12 years of age consult a physician Apply a thin layer to affected area not more than 3 to 4 times daily Gently massage into the skin until fully absorbed Wash hands with soap and water after each application to avoid spreading to the eyes or other sensitive mucous membranes
Other information
It is recommended to test for skin sensitivity prior to use. Apply product to a small area, follow the directions and wait 24 hours. Proceed with application if no adverse reaction. A burning sensation may occur upon inital application but generally disappears with continued use. For a severe burning discomfort, remove excess product with a soft cloth and cooking oil. If applying product to hands, wait 30 minutes before washing. Avoid water, heat, or direct sunlight while using product. If wearing contact lenses, insert prior to application and avoid touching them after applying product. If eye contact occurs, flush immediately with water.
Inactive ingredients
Water (Aqua), Glycerin, PEG-75 Lanolin, Aloe Barbadensis Leaf Juice, PEG-40 Hydrogenated Castor Oil, Acrylates/C10-30 Alkyl Acrylates Crosspolymer, Triethanolamine, Polysorbate 20, Celosia Argentea Seed Extract, Ginkgo Biloba Extract, Panax Ginseng Root Extract, Medicago Sativa (Alfalfa) Extract, Cymbopogon Schoenanthus Extract, Emu Oil, Dimethyl Sulfone, Tocopheryl Acetate, Glucosamine HCl, Propylene Glycol, DMDM Hydantoin, Methylparaben, Propylparaben.
Questions or comments?
Call 1-888-373-8831 or visit www.conceptlabs.org
Manufactured by: #CL9775
Concept Laboratories, Inc.
Chicago, IL 60642 (USA) NDC 64058-813-49
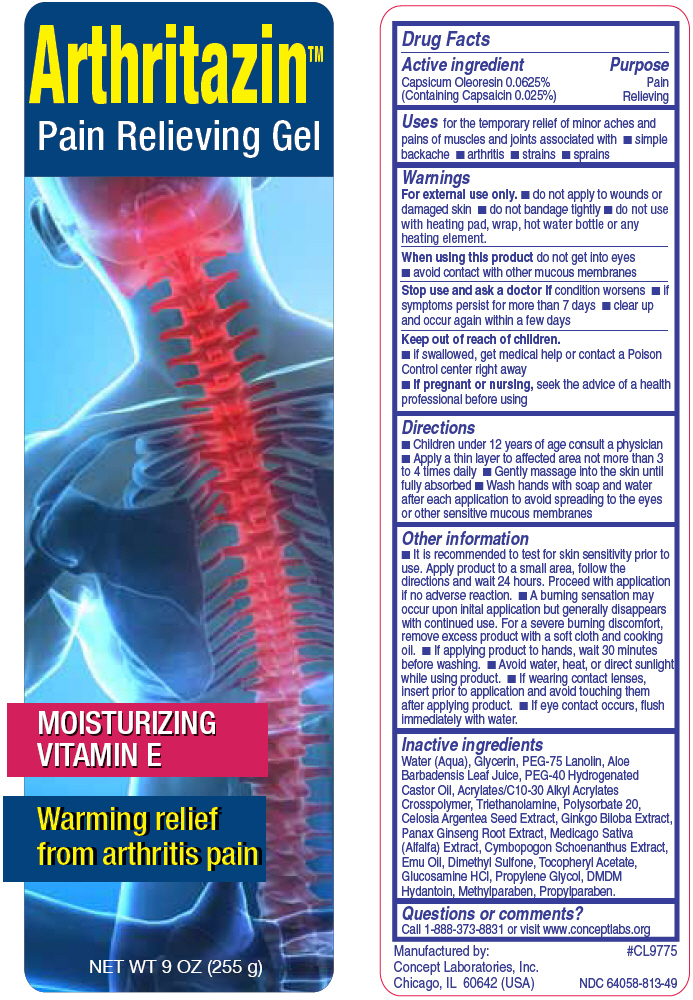
PRINCIPAL DISPLAY PANEL - 255 g Bottle Label
ArthritazinTM
Pain Relieving Gel
Moisturizing Vitamin E
Warming relief from arthritis pain
NET WT 9 OZ (255 g)
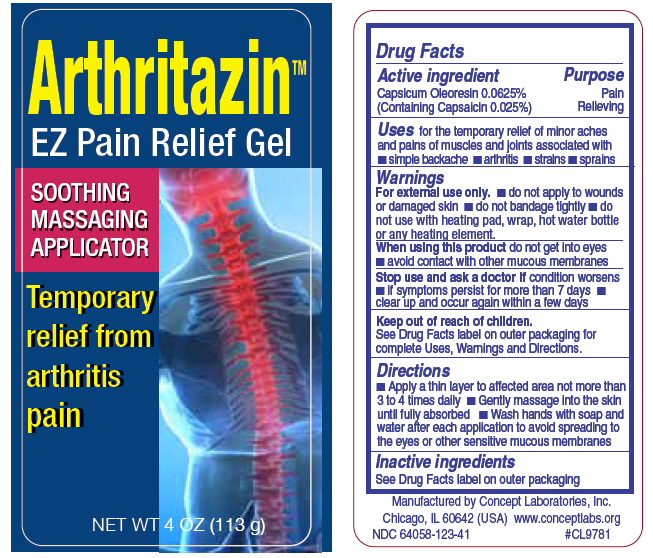
PRINCIPAL DISPLAY PANEL - 113 g Bottle Label
ArthritazinTM
EZ Pain Relief Gel
Soothing Massaging Applicator
Temporary relief from arthritis pain
NET WT 4 OZ (113 g)
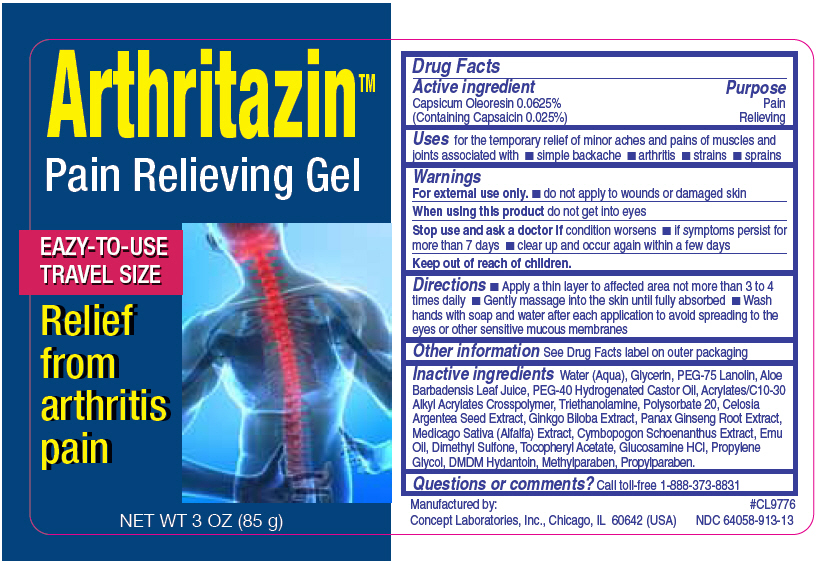
PRINCIPAL DISPLAY PANEL - 85 g Bottle Label
ArthritazinTM
Pain Relieving Gel
Eazy to use travel size
Relief from arthritis pain
NET WT 3 OZ (85 g)
Concept Laboratories, Inc.