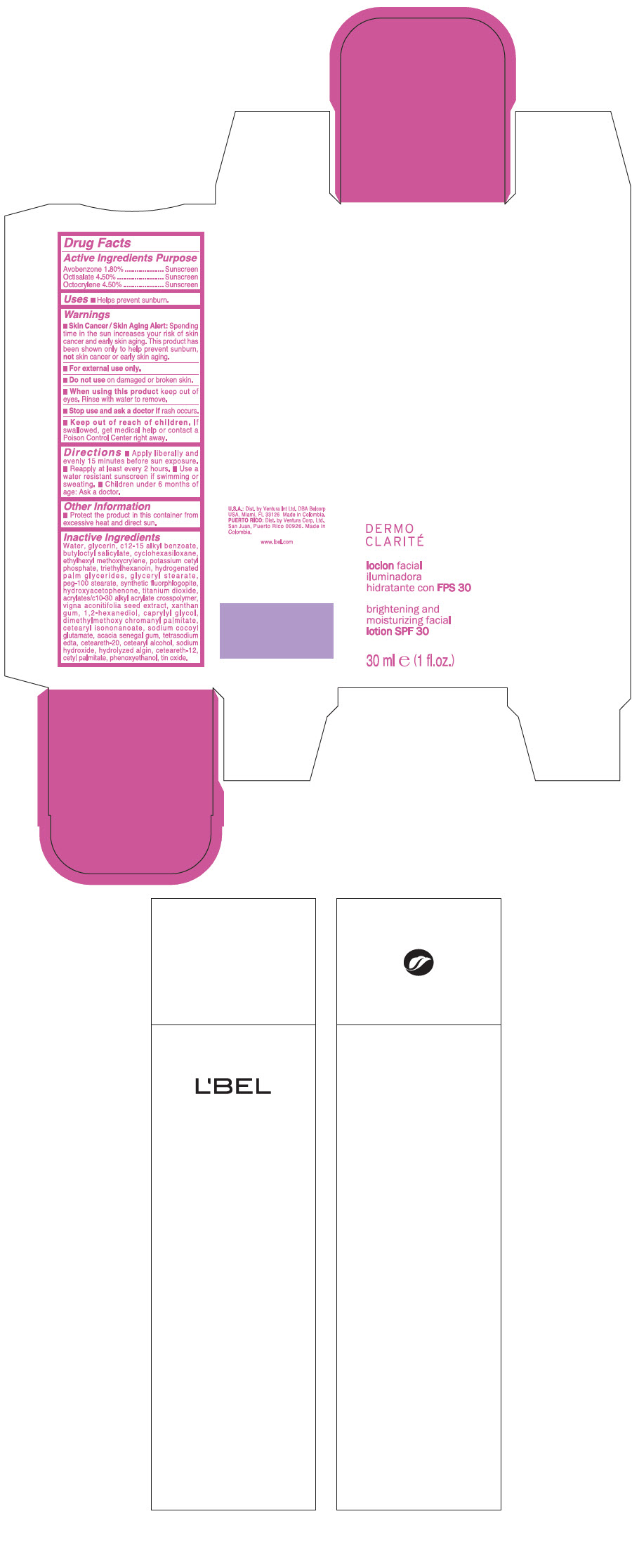
Label: LBEL DERMO CLARITE BRIGHTENING AND MOISTURIZING FACIAL SPF 30- avobenzone, octisalate, and octocrylene lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 43596-0088-1, 43596-0088-2 - Packager: Ventura Corporation LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 17, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
WATER, GLYCERIN, C12-15 ALKYL BENZOATE, BUTYLOCTYL SALICYLATE, CYCLOHEXASILOXANE, ETHYLHEXYL METHOXYCRYLENE, POTASSIUM CETYL PHOSPHATE, TRIETHYLHEXANOIN, HYDROGENATED PALM GLYCERIDES, GLYCERYL STEARATE, PEG-100 STEARATE, SYNTHETIC FLUORPHLOGOPITE, HYDROXYACETOPHENONE, TITANIUM DIOXIDE, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, VIGNA ACONITIFOLIA SEED EXTRACT, XANTHAN GUM, 1,2-HEXANEDIOL, CAPRYLYL GLYCOL, DIMETHYLMETHOXY CHROMANYL PALMITATE, CETEARYL ISONONANOATE, SODIUM COCOYL GLUTAMATE, ACACIA SENEGAL GUM, TETRASODIUM EDTA, CETEARETH-20, CETEARYL ALCOHOL, SODIUM HYDROXIDE, HYDROLYZED ALGIN, CETEARETH-12, CETYL PALMITATE, PHENOXYETHANOL, TIN OXIDE
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 30 ml Jar Carton
-
INGREDIENTS AND APPEARANCE
LBEL DERMO CLARITE BRIGHTENING AND MOISTURIZING FACIAL SPF 30
avobenzone, octisalate, and octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43596-0088 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 0.018 g in 1 mL Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 0.045 g in 1 mL Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 0.045 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CYCLOMETHICONE 6 (UNII: XHK3U310BA) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) TRIETHYLHEXANOIN (UNII: 7K3W1BIU6K) HYDROGENATED PALM GLYCERIDES (UNII: YCZ8EM144Q) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) 4-HYDROXY ACETOPHENONE (UNII: G1L3HT4CMH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XANTHAN GUM (UNII: TTV12P4NEE) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHYLMETHOXY CHROMANYL PALMITATE (UNII: 5G222ZDK7U) CETEARYL ISONONANOATE (UNII: P5O01U99NI) SODIUM COCOYL GLUTAMATE (UNII: BMT4RCZ3HG) ACACIA (UNII: 5C5403N26O) EDETATE SODIUM (UNII: MP1J8420LU) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) SODIUM HYDROXIDE (UNII: 55X04QC32I) CETEARETH-12 (UNII: 7V4MR24V5P) CETYL PALMITATE (UNII: 5ZA2S6B08X) PHENOXYETHANOL (UNII: HIE492ZZ3T) STANNIC OXIDE (UNII: KM7N50LOS6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43596-0088-2 1 in 1 BOX 08/08/2018 1 NDC:43596-0088-1 30 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 08/08/2018 Labeler - Ventura Corporation LTD (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(43596-0088)