CLINDAMAX- clindamycin phosphate cream
PharmaDerm a division of Fougera Pharmaceuticals Inc.
----------
CLINDAMAX® VAGINAL CREAM
(clindamycin phosphate vaginal cream USP, 2%)
equivalent to 2% clindamycin
DESCRIPTION
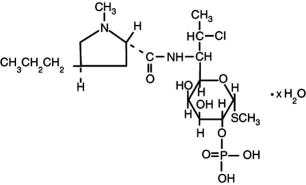
Clindamycin phosphate is a water soluble ester of the semi-synthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent antibiotic lincomycin. The chemical name for clindamycin phosphate is methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl- trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-α-D-galacto-octopyranoside 2-(dihydrogen phosphate). It has a molecular weight of 504.96, and the molecular formula is C18H34ClN2O8 PS. The structural formula is represented below:
ClindaMax® Vaginal Cream (clindamycin phosphate vaginal cream USP, 2%), is a semi-solid, white cream, which contains 2% clindamycin phosphate, USP, at a concentration equivalent to 20 mg clindamycin per gram. The pH of the cream is between 3.0 and 6.0. The cream also contains benzyl alcohol, cetostearyl alcohol, cetyl palmitate, mineral oil, polysorbate 60, propylene glycol, sorbitan monostearate, and stearic acid.
Each applicatorful of 5 grams of vaginal cream contains approximately 100 mg of clindamycin phosphate.
CLINICAL PHARMACOLOGY
Following a once a day intravaginal dose of 100 mg of clindamycin phosphate vaginal cream 2%, administered to 6 healthy female volunteers for 7 days, approximately 5% (range 0.6% to 11%) of the administered dose was absorbed systemically. The peak serum clindamycin concentration observed on the first day averaged 18 ng/mL (range 4 to 47 ng/mL) and on day 7 it averaged 25 ng/mL (range 6 to 61 ng/mL). These peak concentrations were attained approximately 10 hours post-dosing (range 4-24 hours).
Following a once a day intravaginal dose of 100 mg of clindamycin phosphate vaginal cream 2%, administered for 7 consecutive days to 5 women with bacterial vaginosis, absorption was slower and less variable than that observed in healthy females. Approximately 5% (range 2% to 8%) of the dose was absorbed systemically. The peak serum clindamycin concentration observed on the first day averaged 13 ng/mL (range 6 to 34 ng/mL) and on day 7 it averaged 16 ng/mL (range 7 to 26 ng/mL). These peak concentrations were attained approximately 14 hours post-dosing (range 4-24 hours).
There was little or no systemic accumulation of clindamycin after repeated vaginal dosing of clindamycin phosphate vaginal cream 2%. The systemic half-life was 1.5 to 2.6 hours.
MICROBIOLOGY
Clindamycin inhibits bacterial protein synthesis at the level of the bacterial ribosome. The antibiotic binds preferentially to the 50S ribosomal subunit and affects the process of peptide chain initiation. Although clindamycin phosphate is inactive in vitro, rapid in vivo hydrolysis converts this compound to the antibacterially active clindamycin.
Culture and sensitivity testing of bacteria are not routinely performed to establish the diagnosis of bacterial vaginosis. (See INDICATIONS AND USAGE.) Standard methodology for the susceptibility testing of the potential bacterial vaginosis pathogens, Gardnerella vaginalis, Mobiluncus spp., or Mycoplasma hominis, has not been defined. Nonetheless, clindamycin is an antimicrobial agent active in vitro against most strains of the following organisms that have been reported to be associated with bacterial vaginosis:
|
Bacteroides spp. |
Mycoplasma hominis |
|
Peptostreptococcus spp. |
Gardnerella vaginalis |
|
Mobiluncus spp. |
INDICATIONS AND USAGE
ClindaMax® Vaginal Cream, is indicated in the treatment of bacterial vaginosis (formerly referred to as Haemophilus vaginitis, Gardnerella vaginitis, nonspecific vaginitis, Corynebacterium vaginitis, or anaerobic vaginosis). ClindaMax® Vaginal Cream, can be used to treat non-pregnant women and pregnant women during the second and third trimester. (See CLINICAL STUDIES.)
NOTE: For purposes of this indication, a clinical diagnosis of bacterial vaginosis is usually defined by the presence of a homogeneous vaginal discharge that (a) has a pH of greater than 4.5, (b) emits a "fishy" amine odor when mixed with a 10% KOH solution, and (c) contains clue cells on microscopic examination. Gram's stain results consistent with a diagnosis of bacterial vaginosis include (a) markedly reduced or absent Lactobacillus morphology, (b) predominance of Gardnerella morphotype, and (c) absent or few white blood cells.
Other pathogens commonly associated with vulvovaginitis, e.g., Trichomonas vaginalis, Chlamydia trachomatis, N. gonorrhoeae, Candida albicans, and Herpes simplex virus should be ruled out.
CONTRAINDICATIONS
ClindaMax® Vaginal Cream, is contraindicated in individuals with a history of hypersensitivity to clindamycin, lincomycin, or any of the components of this vaginal cream. ClindaMax® Vaginal Cream, is also contraindicated in individuals with a history of regional enteritis, ulcerative colitis, or a history of "antibiotic-associated" colitis.
WARNINGS
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including clindamycin, and may range in severity from mild to life-threatening. Orally and parenterally administered clindamycin has been associated with severe colitis which may end fatally. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of orally and parenterally administered clindamycin, as well as with topical (dermal) formulations of clindamycin. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of clindamycin, even when administered by the vaginal route, because approximately 5% of the clindamycin dose is systemically absorbed from the vagina.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is a primary cause of "antibiotic-associated" colitis.
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to discontinuation of the drug alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against Clostridium difficile colitis.
Onset of pseudomembranous colitis symptoms may occur during or after antimicrobial treatment.
PRECAUTIONS
General
ClindaMax® Vaginal Cream, contains ingredients that will cause burning and irritation of the eye. In the event of accidental contact with the eye, rinse the eye with copious amounts of cool tap water.
The use of ClindaMax® Vaginal Cream may result in the overgrowth of nonsusceptible organisms in the vagina. In clinical studies involving 600 non-pregnant women who received treatment for 3 days, Candida albicans was detected, either symptomatically or by culture, in 8.8% of patients. In 9% of the patients, vaginitis was recorded. In clinical studies involving 1325 non-pregnant women who received treatment for 7 days, Candida albicans was detected, either symptomatically or by culture, in 10.5% of patients. Vaginitis was recorded in 10.7% of the patients. In 180 pregnant women who received treatment for 7 days, Candida albicans was detected, either symptomatically or by culture, in 13.3% of patients. In 7.2% of the patients, vaginitis was recorded. Candida albicans, as reported here, includes the terms: vaginal moniliasis and moniliasis (body as a whole). Vaginitis includes the terms: vulvo-vaginal disorder, vulvovaginitis, vaginal discharge, trichomonal vaginitis, and vaginitis.
Information for the Patient
The patient should be instructed not to engage in vaginal intercourse, or use other vaginal products (such as tampons or douches) during treatment with this product.
The patient should also be advised that this cream contains mineral oil that may weaken latex or rubber products such as condoms or vaginal contraceptive diaphragms. Therefore, use of such products within 72 hours following treatment with ClindaMax®Vaginal Cream (clindamycin phosphate vaginal cream 2%), is not recommended.
Drug Interactions
Clindamycin has been shown to have neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. Therefore, it should be used with caution in patients receiving such agents.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term studies in animals have not been performed with clindamycin to evaluate carcinogenic potential. Genotoxicity tests performed included a rat micronucleus test and an Ames test. Both tests were negative. Fertility studies in rats treated orally with up to 300 mg/kg/day (31 times the human exposure based on mg/m2 ) revealed no effects on fertility or mating ability.
Pregnancy: Teratogenic effects
Pregnancy Category B
There are no adequate and well-controlled studies in pregnant women during the first trimester of pregnancy. This drug should be used during the first trimester of pregnancy only if clearly needed.
Clindamycin phosphate vaginal cream 2% has been studied in pregnant women during the second trimester. In women treated for seven days, abnormal labor was reported in 1.1% of patients who received clindamycin phosphate vaginal cream 2% compared with 0.5% of patients who received placebo.
Reproduction studies have been performed in rats and mice using oral and parenteral doses of clindamycin up to 600 mg/kg/day (62 and 25 times, respectively, the maximum human exposure based on mg/m2 ) and have revealed no evidence of harm to the fetus due to clindamycin. In one mouse strain, cleft palates were observed in treated fetuses; this outcome was not produced in other mouse strains or in other species and is, therefore, considered to be a strain specific effect.
See INDICATIONS AND USAGE; PRECAUTIONS, General; and ADVERSE REACTIONS.
Nursing Mothers
Clindamycin has been detected in human milk after oral or parenteral administration. It is not known if clindamycin is excreted in human milk following the use of vaginally administered clindamycin phosphate.
Because of the potential for serious adverse reactions in nursing infants from clindamycin phosphate, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Geriatric Use
Clinical studies for clindamycin vaginal cream 2% did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
ADVERSE REACTIONS
Clinical trials
Non-pregnant Women: In clinical trials involving non-pregnant women, 1.8% of 600 patients who received treatment with clindamycin phosphate vaginal cream 2% for 3 days and 2.7% of 1325 patients who received treatment for 7 days discontinued therapy due to drug-related adverse events. Medical events judged to be related, probably related, possibly related, or of unknown relationship to vaginally administered clindamycin phosphate vaginal cream 2%, were reported for 20.7% of the patients receiving treatment for 3 days and 21.3% of the patients receiving treatment for 7 days. Events occurring in ≥1% of patients receiving clindamycin phosphate vaginal cream 2% are shown in Table 1.
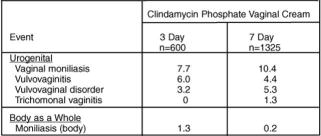
Table 1- Events Occurring in ≥1% of Non-pregnant Patients Receiving Clindamycin Phosphate Vaginal Cream 2%
Other events occurring in <1% of the clindamycin vaginal cream 2% groups include:
Urogenital system: vaginal discharge, metrorrhagia, urinary tract infection, endometriosis, menstrual disorder, vaginitis/vaginal infection, and vaginal pain.
Body as a whole: localized abdominal pain, generalized abdominal pain, abdominal cramps, halitosis, headache, bacterial infection, inflammatory swelling, allergic reaction, and fungal infection.
Digestive system: nausea, vomiting, constipation, dyspepsia, flatulence, diarrhea, and gastrointestinal disorder.
Endocrine system: hyperthyroidism.
Central nervous system: dizziness and vertigo.
Respiratory system: epistaxis.
Skin: pruritus (non-application site), moniliasis, rash, maculopapular rash, erythema, and urticaria.
Special senses: taste perversion.
Pregnant Women: In a clinical trial involving pregnant women during the second trimester, 1.7% of 180 patients who received treatment for 7 days discontinued therapy due to drug-related adverse events. Medical events judged to be related, probably related, possibly related, or of unknown relationship to vaginally administered clindamycin phosphate vaginal cream 2%, were reported for 22.8% of pregnant patients. Events occurring in ≥1% of patients receiving either clindamycin phosphate vaginal cream 2% or placebo are shown in Table 2.
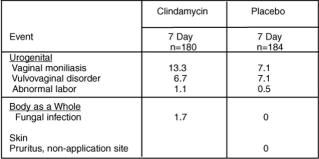
Table 2- Events Occurring in ≥1% of Pregnant Patients Receiving Clindamycin Phosphate Vaginal Cream 2% or Placebo
Other events occurring in <1% of the clindamycin vaginal cream 2% group include:
Urogenital system: dysuria, metrorrhagia, vaginal pain, and trichomonal vaginitis.
Body as a whole: upper respiratory infection.
Skin: pruritus (topical application site) and erythema.
Other clindamycin formulations:
Clindamycin vaginal cream affords minimal peak serum levels and systemic exposure (AUCs) of clindamycin compared to 100 mg oral clindamycin dosing. Although these lower levels of exposure are less likely to produce the common reactions seen with oral clindamycin, the possibility of these and other reactions cannot be excluded presently. Data from well-controlled trials directly comparing clindamycin administered orally to clindamycin administered vaginally are not available.
The following adverse reactions and altered laboratory tests have been reported with the oral or parenteral use of clindamycin:
Gastrointestinal: Abdominal pain, esophagitis, nausea, vomiting, and diarrhea. (See WARNINGS.)
Hematopoietic: Transient neutropenia (leukopenia), eosinophilia, agranulocytosis, and thrombocytopenia have been reported. No direct etiologic relationship to concurrent clindamycin therapy could be made in any of these reports.
Hypersensitivity Reactions: Maculopapular rash and urticaria have been observed during drug therapy. Generalized mild to moderate morbilliform-like skin rashes are the most frequently reported of all adverse reactions. Rare instances of erythema multiforme, some resembling Stevens-Johnson syndrome, have been associated with clindamycin. A few cases of anaphylactoid reactions have been reported. If a hypersensitivity reaction occurs, the drug should be discontinued.
Liver: Jaundice and abnormalities in liver function tests have been observed during clindamycin therapy.
Musculoskeletal: Rare instances of polyarthritis have been reported.
Renal: Although no direct relationship of clindamycin to renal damage has been established, renal dysfunction as evidenced by azotemia, oliguria, and/or proteinuria has been observed in rare instances.
OVERDOSAGE
Vaginally applied ClindaMax® Vaginal Cream could be absorbed in sufficient amounts to produce systemic effects. (See WARNINGS.)
DOSAGE AND ADMINISTRATION
The recommended dose is one applicatorful of ClindaMax® Vaginal Cream, (5 grams containing approximately 100 mg of clindamycin phosphate) intravaginally, preferably at bedtime, for 3 or 7 consecutive days in non-pregnant patients and for 7 consecutive days in pregnant patients. (See CLINICAL STUDIES.)
HOW SUPPLIED
ClindaMax® Vaginal Cream (clindamycin phosphate vaginal cream USP, 2%) is a white to off-white cream having a slight odor and is supplied as follows:
|
40 g tube (with 7 disposable applicators) |
NDC 0462-0277-40 |
Store at 20° to 25°C (68° to 77° F)[see USP Controlled Room Temperature].
CLINICAL STUDIES
In two clinical studies involving 674 evaluable non-pregnant women with bacterial vaginosis comparing clindamycin phosphate vaginal cream 2% for 3 or 7 days, the clinical cure rates, determined at 1 month posttherapy, ranged from 72% to 81% for the 3-day treatment and 84% to 86% for the 7-day treatment.
|
Clindamycin Phosphate 3 Day |
Clindamycin Phosphate 7 Day |
|||
|
U.S. Study |
94/131 |
72% |
110/128 |
86% |
|
European Study |
161/199 |
81% |
181/216 |
84% |
In a clinical study involving 249 evaluable pregnant patients in the second and third trimester treated for 7 days, the clinical cure rate, determined at 1 month posttherapy, was 60% (77/129) in the clindamycin arm and 9% (11/120) for the vehicle arm. The determination of clinical cure was based on the absence of a "fishy" amine odor when the vaginal discharge was mixed with a 10% KOH solution and the absence of clue cells on microscopic examination.
- PharmaDerm®
A division of Nycomed US Inc.
Melville, NY 11747 USA
www.pharmaderm.com
I8277B
R3/08
#305
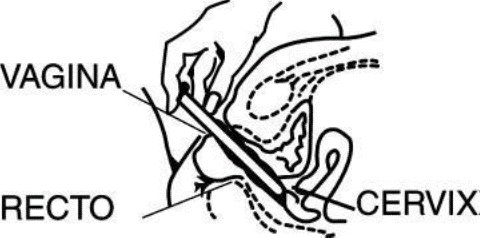
DIRECTIONS FOR USE
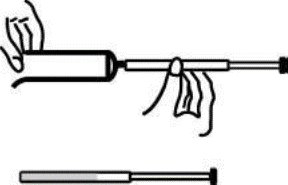
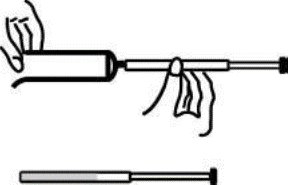
7 Disposable plastic applicators are provided with this package. They are designed to allow proper vaginal administration of the cream.
Remove cap from cream tube. Screw a plastic applicator on the threaded end of the tube.
Rolling tube from the bottom, squeeze gently and force the medication into the applicator.
The applicator is filled when the plunger reaches its predetermined stopping point.
Unscrew the applicator from the tube and replace the cap.
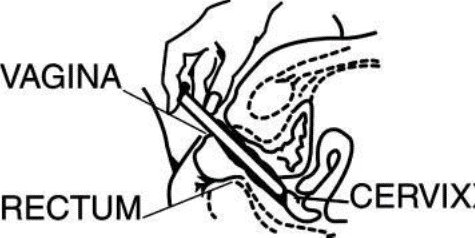
While lying on your back, firmly grasp the applicator barrel and insert into vagina as far as possible without causing discomfort.
Slowly push the plunger until it stops. Carefully withdraw applicator from vagina, and discard applicator.
REMEMBER TO APPLY ONE APPLICATORFUL EACH NIGHT BEFORE BEDTIME, OR AS PRESCRIBED BY YOUR DOCTOR.
INSTRUCCIONES PARA LA PACIENTE
Este envase contiene 7 aplicadores de plástico desechables. Los aplicadores están diseñados para la adminstración apropiada de la crema en la vagina.
Remueva la tapa del tubo de crema y enrosque el aplicador de plástico al tubo.
Exprima el tubo suavemente desde el extremo inferior y fuerce el medicamento al aplicador.
El aplicador estará lleno cuando el émbolo llega a su máxima longitud.
Desenrosque el aplicador del tubo y vuelva a poner la tapa.
Acuéstese de espada y agarrando firemente el aplicador, introdúzcalo en la vagina tanto como sea posible sin causar molestias.
Empuje lentamente el émbolo hasta que se detenga.
Saque el aplicador cuidadosamente de la vagina y descártelo.
RECUERDE APLICARSE UN APLICADOR LLENO TODAS LAS NOCHES AL ACOSTARSE, O DE ACUDERDO CON LAS INDICACIONES DE SU MEDICO.
| CLINDAMAX
clindamycin phosphate cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - PharmaDerm a division of Fougera Pharmaceuticals Inc. (043838424) |