LACTATED RINGERS- sodium chloride, sodium lactate, potassium chloride and calcium chloride solution, for slush
Baxter Healthcare Corporation
----------
Lactated Ringer’s Irrigation
Baxter Sterile Container System
DESCRIPTION
Lactated Ringer’s Irrigation in the Baxter Sterile Container System is a sterile, nonpyrogenic, isotonic solution for the preparation of slushed solution. It contains no antimicrobial agents. Composition, osmolarity, pH, ionic concentration and caloric content are shown in Table 1.
|
Size (mL) |
Composition (g/L) |
Osmolarity (mOsmol/L) (calc) |
pH |
Ionic Composition (mEq/L) |
Caloric Content (kcal/L) |
||||||||
|
Sodium Chloride, USP, (NaCl) |
Sodium Lactate, (C3H5NaO3) |
Potassium Chloride, USP, (KCl) |
Calcium Chloride, USP (CaCl2·2H2O) |
Sodium |
Potassium |
Calcium |
Chloride |
Lactate |
|||||
|
Lactated Ringer’s Irrigation |
1000 |
6 |
3.1 |
0.3 |
0.2 |
273 |
6.5 |
130 |
4 |
3 |
109 |
28 |
9 |
The Baxter Sterile Container System is designed to provide a slush container with a sterile exterior surface for use within the surgical field. Within the overwrap, the unit is packaged in a dispensing bag which maintains the sterility of this surface.
The flexible plastic slush container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic). The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexyl phthalate (DEHP), up to 5 parts per million. However, the safety of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as by culture toxicity studies.
CLINICAL PHARMACOLOGY
Slushed solution is used to induce regional hypothermia in conditions such as certain open heart and kidney surgical procedures by direct application of slushed solution. The objective of regional hypothermia is to reduce cellular metabolic activity so that body tissues can tolerate a period of total or relative ischemia thereby inhibiting the formation and buildup of destructive autolytic enzymes and anaerobic by-products usually produced and accumulated in ischemic tissues.
INDICATIONS AND USAGE
Slushed solution is used to create regional hypothermia in order to reduce and minimize manifestations of warm-temperature ischemia. Temperature probes may be used to determine requirements for replacement or addition of slushed solution.
PRECAUTIONS
Avoid prolonged direct contact between ice crystals and body tissues.
Experience in use of slushed solutions in pediatrics is limited.
Carcinogenesis, mutagenesis, impairment of fertility
Studies performed with slushed Lactated Ringer’s Irrigation have not been performed to evaluate carcinogenic potential, mutagenic potential, or effects on fertility.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Animal reproduction studies have not been conducted with slushed Lactated Ringer’s Irrigation. It is also not known whether slushed Lactated Ringer’s Irrigation can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Slushed Lactated Ringer’s Irrigation should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when slushed Lactated Ringer’s irrigation is administered to a nursing mother.
Pediatric Use
Safety and effectiveness of slushed Lactated Ringer’s Irrigation in pediatric patients have not been established by adequate and well controlled trials; however, the use of electrolyte solutions in the pediatric population is referenced in the medical literature.
The warnings, precautions and adverse reactions identified in the label copy should be observed in the pediatric population.
Do not administer unless seals of outer dispensing bag are intact.
DOSAGE AND ADMINISTRATION
The volume of slushed solution required will vary with patient’s age, clinical condition, cooling effect desired and duration of cooling effect desired, according to physician’s instructions.
HOW SUPPLIED
Lactated Ringer’s Irrigation in the Baxter Sterile Container System is supplied as follows:
|
Code |
Size (mL) |
NDC |
DIN |
|
2B7233 |
1000 |
0338-0118-44 |
799971 |
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Avoid freezing except during controlled slushing procedure. It is recommended that this product be stored at room temperature (25ºC); brief exposure up to 40ºC does not adversely affect this product.
DIRECTIONS FOR USE OF THE BAXTER STERILE CONTAINER SYSTEM
Guidelines for Preparation of Slush
The following instructions are intended only as guidelines for the preparation of slush using the Baxter Sterile Container System.
The specific procedure necessary to achieve the desired slush consistency will depend on the type of freezer used, location of the product in the freezer, and the utilization of freezer capacity.
- 1.
- Remove product from shipping carton and place in freezer at a temperature between minus 4°C and minus 15°C for 2 to 6 hours.
Note: A pre-cooled product will require less time to slush. - 2.
- During the freezing process, remove product from freezer periodically and carefully massage to break up large frozen chunks of solution.
- 3.
- Return product to freezer and repeat procedure until desired slush consistency is obtained.
To Open: Use Aseptic Technique
A. To prepare unit outside the surgical field.
- 1.
- Tear overwrap down side at slit and remove dispensing bag which contains the slush container.
- 2.
- Allow unit to stand at room temperature for approximately 10 minutes to allow container material to regain flexibility.
- 3.
- Check that all the seals of the dispensing bag are intact including the tamper evident button. If any broken seals or holes are detected, discard unit as sterility may be impaired.
- 4.
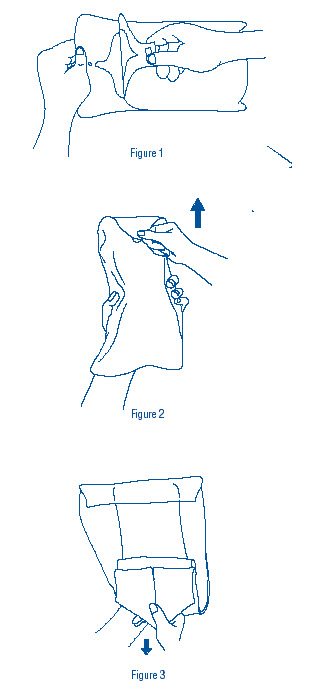
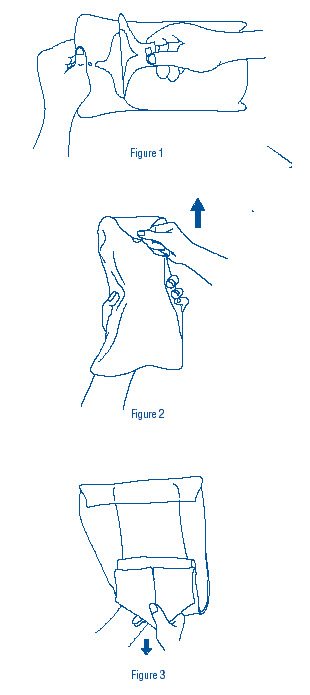
- Grasp tab of dispensing bag and peel open (Figure 1). Remove wrapped inner slush container.
- 5.
- Grasp point of sterile transfer wrap (Figure 2), and pull forward then back to expose sterile slush container (Figure 3). Caution should be exercised to avoid touch contamination.
B. To transfer sterile slush container to the surgical field.
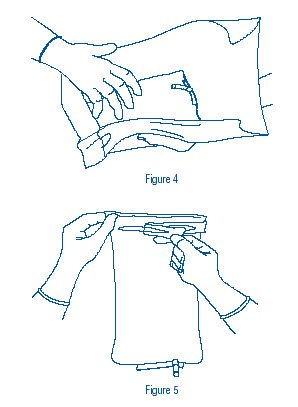
- 1.
- Using sterile gloved hands, remove slush container from the sterile transfer wrap (Figure 4). Place on surgical field or use immediately.
- 2.
- Grasp tab at top of slush container and peel directly across the width of the container with a steady motion (Figure 5).
- 3.
- Slush is ready for dispensing.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Printed in USA
Distributed in Canada by
Baxter Corporation
Toronto, Ontario, Canada
©Copyright 1984, 1988, 1995, Baxter Healthcare Corporation.
All rights reserved.
07-19-04-502
Rev. October 2003
BAXTER and PL 146 are trademarks of Baxter International Inc.
PRINCIPAL DISPLAY PANEL - PACKAGING LABELING
1000 mL
2B7233
NOT FOR INJECTION
NDC 0338-0188-44
LACTATED RINGER'S
INJECTION
Baxter Sterile Container System
EACH 100 mL CONTAINS 600 mg SODIUM CHLORIDE
USP 310 mg SODIUM LACTATE 30 mg POTASSIUM
CHLORIDE USP 20 mg CALCIUM CHLORIDE USP pH 6.5
(6.0 TO 7.5) mEq/L SODIUM 130 POTASSIUM 4 CALCIUM
3 CHLORIDE 109 LACTATE 28 OSMOLARITY 273
mOsmol/L (CALC) STERILE NONPYROGENIC SINGLE DOSE
CONTAINER
FOR USE ONLY IN SLUSH PREPARATION
FOR REGIONAL HYPOTHERMIA
SEE ACCOMPANYING DIRECTIONS FOR USE
DOSAGE AS DIRECTED BY A PHYSICIAN
CAUTION RX ONLY
BAXTER LOGO
BAXTER HEALTHCARE CORPORATION
DEERFIELD IL 60015 USA
MADE IN USA
PL 146 PLASTIC
BAXTER AND PL 146
ARE TRADEMARKS OF
BAXTER INTERNATIONAL INC
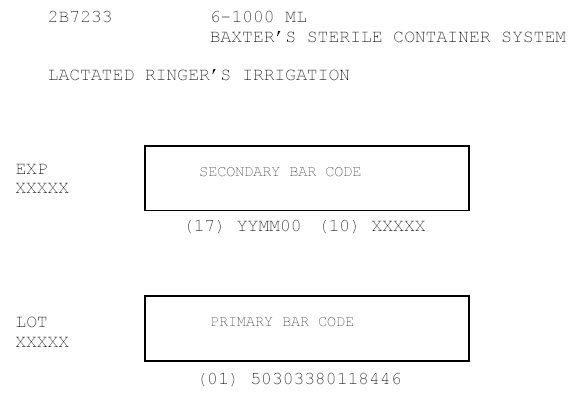
2B7233
6-1000 ML
BAXTER'S STERILE CONTAINER SYSTEM
LACTATED RINGER'S IRRIGATION
EXP
XXXXX
SECONDARY BAR CODE
(17) YYMM00 (10) XXXXX
LOT
XXXXX
PRIMARY BAR CODE
(01) 50303380118446
| LACTATED RINGERS
sodium chloride, sodium lactate, potassium chloride and calcium chloride solution, for slush |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Baxter Healthcare Corporation (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Healthcare Corporation | 059140764 | ANALYSIS(0338-0118) , LABEL(0338-0118) , MANUFACTURE(0338-0118) , PACK(0338-0118) , STERILIZE(0338-0118) | |