STERILE WATER- water injection
Genentech, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Sterile Water for Injection, USP
Genentech
A Member of the Roche Group
IMPORTANT
PRESCRIBING
INFORMATION
May 2018
Subject: Temporary Importation of Sterile Water for Injection (SWFI) Ampules to Address Supply Shortage
Dear Health Care Provider:
In order to address ongoing shortages of Sterile Water for Injection (SWFI), Genentech, Inc. (A Member of the Roche Group) is coordinating with the U.S. FDA Drug Shortage Staff to make available 5 mL SWFI ampules manufactured by Roche's SWFI supplier in Germany.
At this time, no other entity except Genentech is authorized by the FDA to import or distribute Sterile Water for Injection, USP, 5 mL Single-Dose Ampule in the U.S. The FDA has not approved Genentech's Sterile Water for Injection, USP, 5 mL Single-Dose Ampule in the United States.
SWFI presentations most commonly available in the U.S. are in vials. Genentech is temporarily importing SWFI presentations from Germany in 5 mL glass ampules. Pictures of the 5 mL SWFI ampule and label are enclosed at the end of this letter.
Effective immediately, Genentech will offer free of charge a limited supply on an allocation plan of the following:
| Product Name and Description | Size | NDC | Store at |
|---|---|---|---|
| Sterile Water for Injection, USP Sterile, clear and colorless liquid | 5 mL glass ampule | 50242-001-05 | 20°C to 25°C (68°F to 77°F) See USP Controlled Room Temperature |
The dosage and administration instructions provided in the FDA package inserts of the drug being reconstituted should be followed when using the 5 mL SWFI ampules. It is also important to note the following:
- There is a risk of contamination by glass particles when opening the 5 mL SWFI ampules. To minimize particulate contamination:
- Follow standard aseptic technique and withdraw contents of the ampules with a 5 micron filter needle (American Society of Health-System Pharmacists Guidelines on Compounding Sterile Preparations 2014).
- After withdrawing ampule contents with filter needle, change needle before injection.
- The barcode on the SWFI ampule label may not register with U.S. scanning systems. Institutions should manually input the SWFI information into their systems and confirm that the barcode, if scanned, provides correct information. Alternative procedures should be followed to ensure that the correct drug product is being used and administered to individual patients.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
To place an order for 5 mL SWFI ampules, please contact Genentech Customer Service by calling 1-800-551-2231.
If you have any medical questions about the information contained in this letter or the use of the imported 5 mL SWFI ampules, please contact Genentech Medical Communications at 1-800-821-8590.
To report a product complaint concerning the imported 5 mL SWFI ampules, please contact Genentech Product Quality at 1-800-334-0290.
To report an adverse event concerning the imported 5 mL SWFI ampules, please contact Genentech at 1-888-835-2555.
Adverse events or quality problems experienced with the use of the 5 mL SWFI ampules may also be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax:
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Regular mail or fax: Download form at www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
Sincerely,
Lance Baldo, M.D.,
Head of U.S. Medical Affairs
Reference: American Society of Health-System Pharmacists. ASHP Guidelines on Compounding Sterile Preparations. Am J Health-Syst Pharm. 2014; 71:145–66.
| Product Picture and Label |
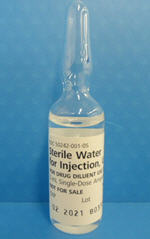

| STERILE WATER
water injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Genentech, Inc. (080129000) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| F. Hoffmann-La Roche Ltd | 485244961 | LABEL(50242-001) | |
