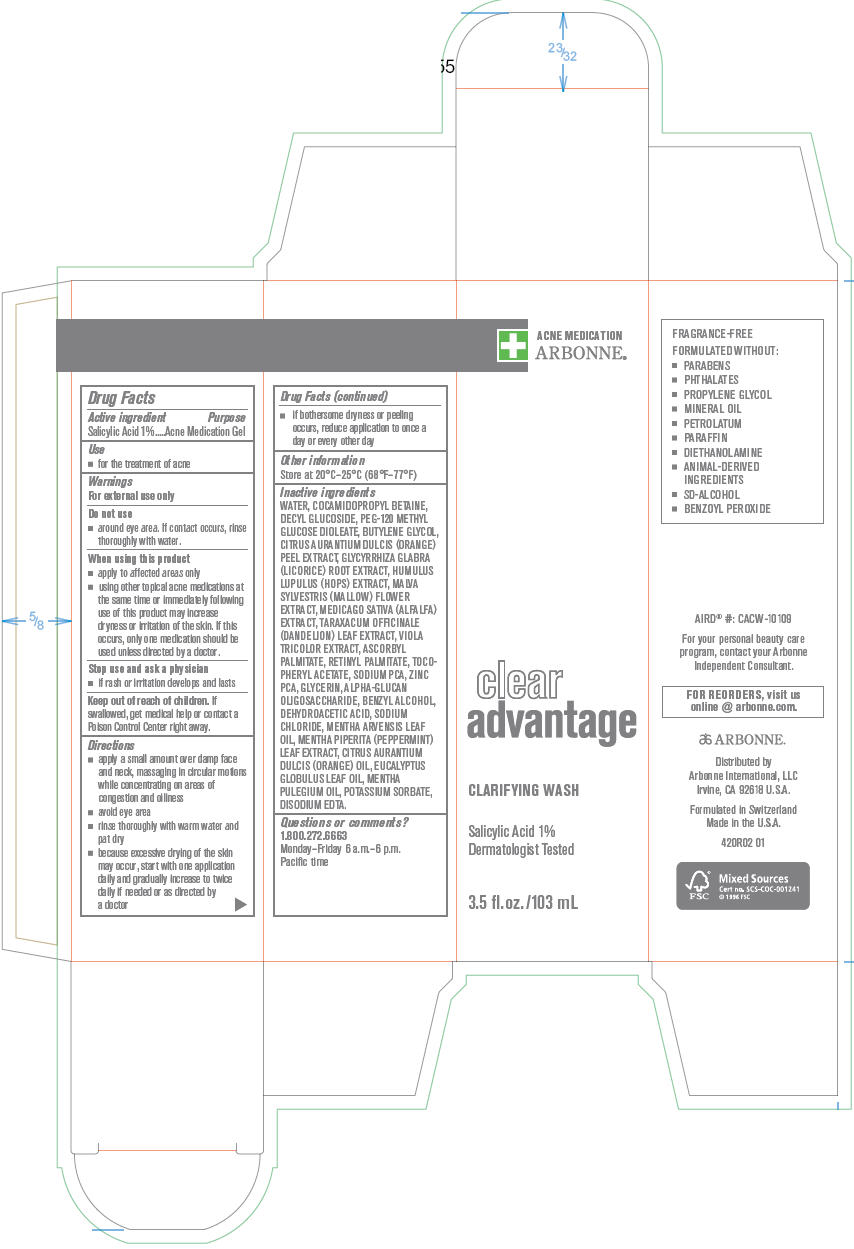
CLEAR ADVANTAGE CLARIFYING WASH- salicylic acid solution
Arbonne International, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Clear Advantage
Clarifying Wash
Warnings
For external use only
Directions
- apply a small amount over damp face and neck, massaging in circular motions while concentrating on areas of congestion and oiliness
- avoid eye area
- rinse thoroughly with warm water and pat dry
- because excessive drying of the skin may occur, start with one application daily and gradually increase to twice daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
Inactive ingredients
WATER, COCAMIDOPROPYL BETAINE, DECYL GLUCOSIDE, PEG-120 METHYL GLUCOSE DIOLEATE, BUTYLENE GLYCOL, CITRUS AURANTIUM DULCIS (ORANGE) PEEL EXTRACT, GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT, HUMULUS LUPULUS (HOPS) EXTRACT, MALVA SYLVESTRIS (MALLOW) FLOWER EXTRACT, MEDICAGO SATIVA (ALFALFA) EXTRACT, TARAXACUM OFFICINALE (DANDELION) LEAF EXTRACT, VIOLA TRICOLOR EXTRACT, ASCORBYL PALMITATE, RETINYL PALMITATE, TOCOPHERYL ACETATE, SODIUM PCA, ZINC PCA, GLYCERIN, ALPHA-GLUCAN OLIGOSACCHARIDE, BENZYL ALCOHOL, DEHYDROACETIC ACID, SODIUM CHLORIDE, MENTHA ARVENSIS LEAF OIL, MENTHA PIPERITA (PEPPERMINT) LEAF EXTRACT, CITRUS AURANTIUM DULCIS (ORANGE) OIL, EUCALYPTUS GLOBULUS LEAF OIL, MENTHA PULEGIUM OIL, POTASSIUM SORBATE, DISODIUM EDTA.
| CLEAR ADVANTAGE
CLARIFYING WASH
salicylic acid solution |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Arbonne International, LLC (021541164) |