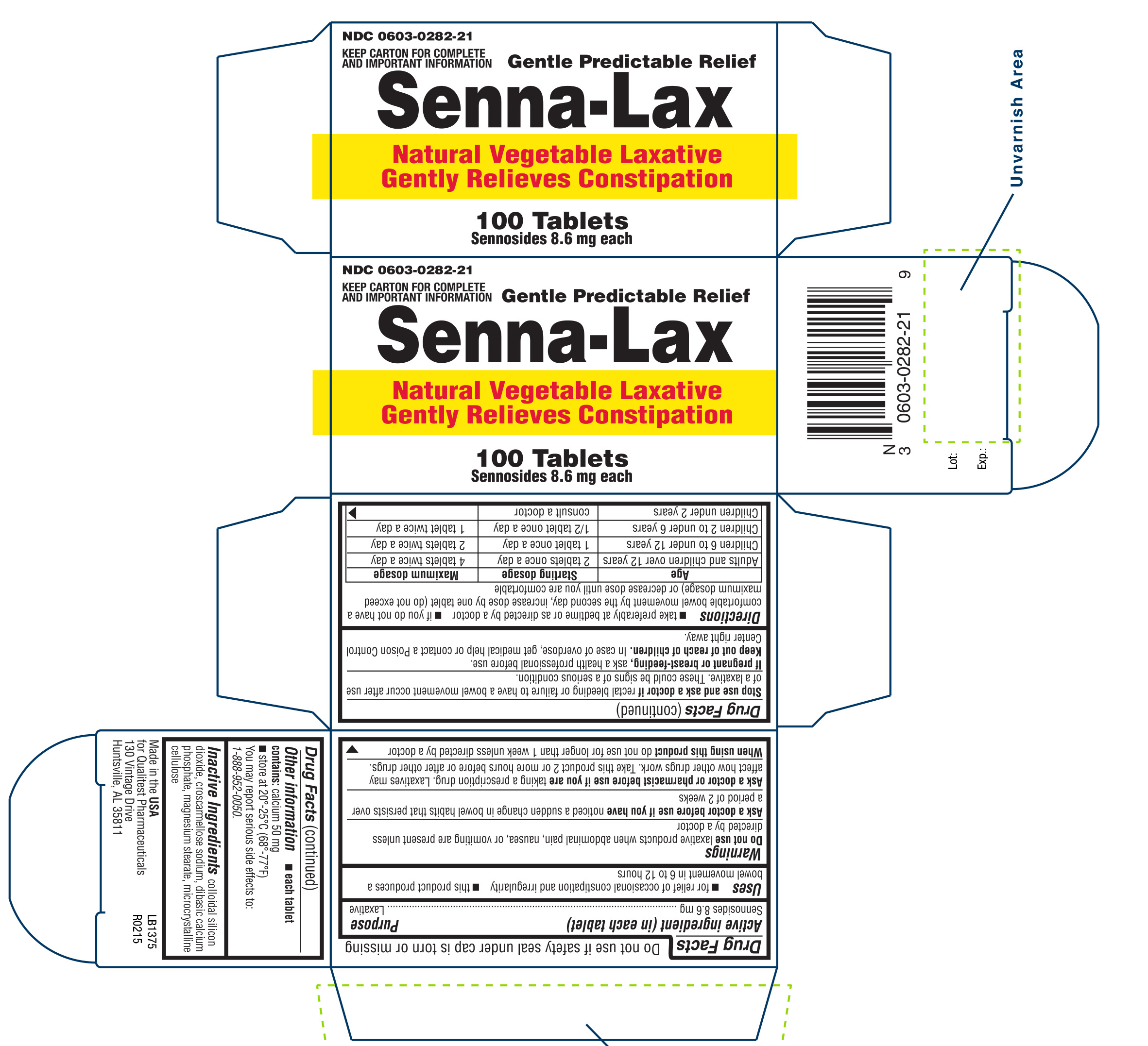
SENNA-LAX- sennosides tablet
Qualitest Pharmaceuticals
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Senna-Lax
USES
- for relief of occasional constipation and irregularity
- this product produces a bowel movement in 6 to 12 hours
WARNINGS
Do not use
laxative products when abdominal pain, nausea, or vomiting are present unless directed by a doctor
Ask a doctor before use if you have
noticed a sudden change in bowel habits that persists over a period of 2 weeks
Ask a doctor or pharmacist if you are
taking a prescription drug. Laxatives may affect how other drugs work. Take this product 2 or more hours before or after other drugs.
DIRECTIONS
- Take preferably at bedtime or as directed by doctor
- If you do not have a comfortable bowel movement by the second day, increase dose by 1 tablet (do not exceed maximum dosage) or decrease dose until you are comfortable
| Age | Starting dosage | Maximum dosage |
| Adults and children over 12 years | 2 tablets once a day | 4 tablets twice a day |
| children 6 to under 12 years | 1 tablet once a day | 2 tablets twice a day |
| children 2 to under 6 years | 1/2 tablet once a day | 1 tablet twice a day |
| children under 2 years | consult a doctor |
OTHER INFORMATION
- each tablet contains: calcium 50 mg
- store at 20°-25°C (68°-77°F)
You may report serious side effects to: 1-888-952-0050.
| SENNA-LAX
sennosides tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Qualitest Pharmaceuticals (011103059) |
| Registrant - Qualitest Pharmaceuticals (011103059) |
Revised: 11/2018
Document Id: 13be4147-756d-4499-b9a1-9ce88efa69ab
Set id: d7dfb02a-41b2-4b0a-9bf6-debf1e69c883
Version: 6
Effective Time: 20181127
Qualitest Pharmaceuticals