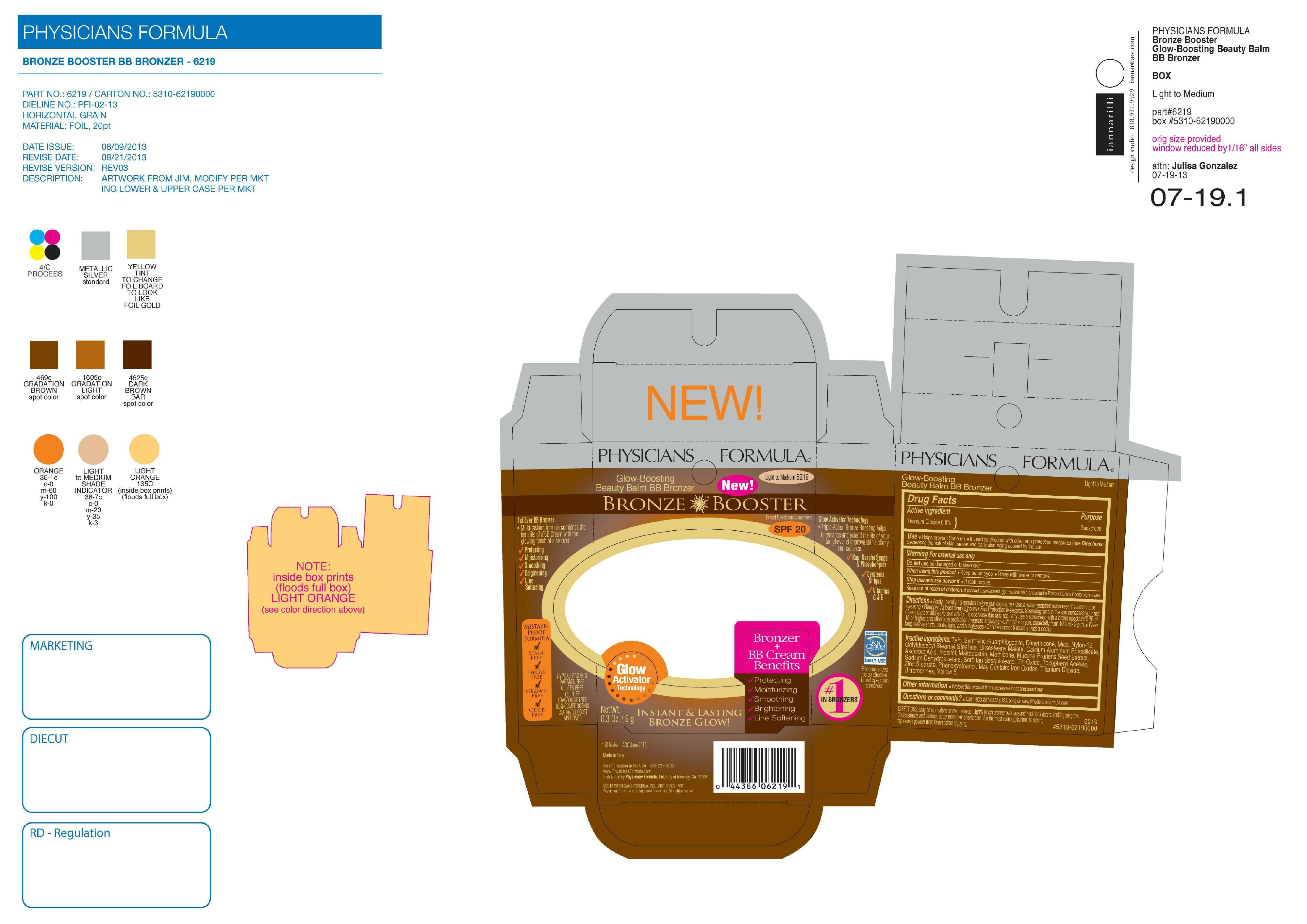
BRONZE BOOSTER BEAUTY BALM BB BRONZER SPF 20- titanium dioxide powder
Physicians Formula Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Bronze Booster BB Bronzer-PF-Tecno
| BRONZE BOOSTER BEAUTY BALM BB BRONZER
SPF 20
titanium dioxide powder |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Physicians Formula Inc (021261805) |
Revised: 12/2019
Document Id: 9a04509d-2982-fd96-e053-2995a90ad178
Set id: d6819879-ff51-45ca-89bc-de2b5e288ebe
Version: 4
Effective Time: 20191218
Physicians Formula Inc