Label: SLEEP-AID CHEWABLES NIGHTTIME- diphenhydramine hcl tablet, chewable
- NDC Code(s): 69842-958-44
- Packager: CVS PHARMACY
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each chewable tablet)
- Purpose
- Uses
-
Warnings
Do not use
- for children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- for children under 12 years of age
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
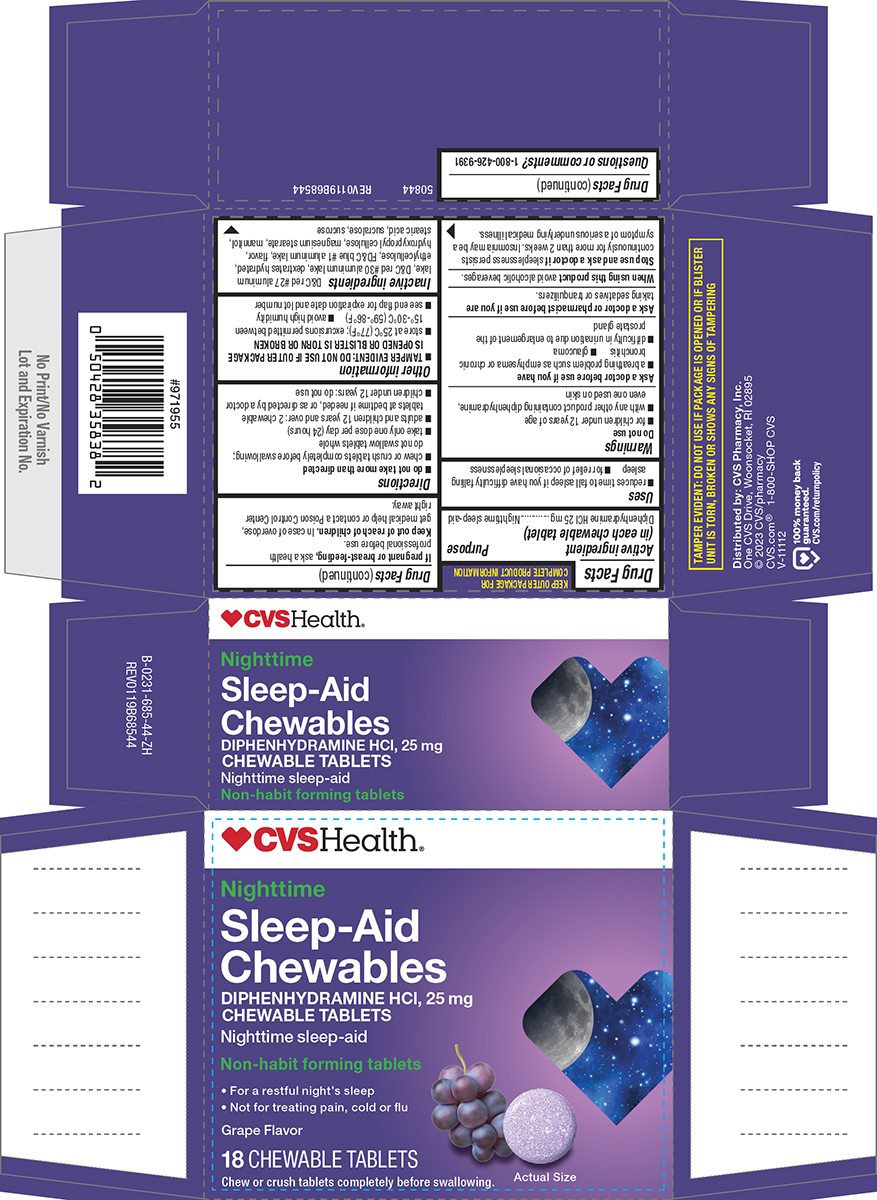
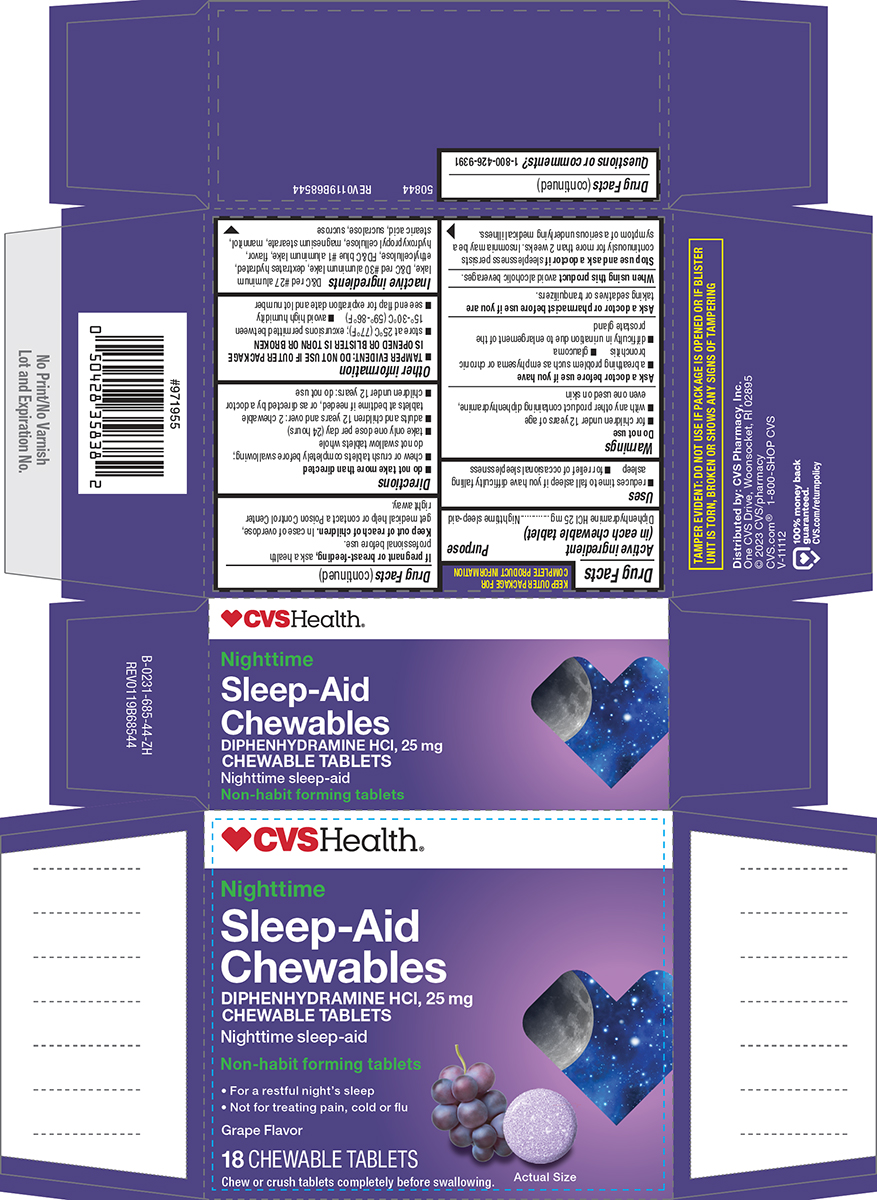
Principal display panel
CVSHealth®
Nighttime
Sleep-Aid
Chewables
DIPHENHYDRAMINE HCl, 25 mg
CHEWABLE TABLETS
Nighttime sleep-aid
Non-habit forming tablets
• For a restful night's sleep
• Not for treating pain, cold or fluGrape Flavor
18 CHEWABLE TABLETS
Chew or crush tablets completely before swallowing.
Actual Size
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING50844 REV0119B68544
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2023 CVS/pharmacy
CVS.com® 1-800-SHOP CVSV-11112
100% money back
guaranteed
CVS.com/returnpolicy.
CVS 44-685 SA
-
INGREDIENTS AND APPEARANCE
SLEEP-AID CHEWABLES NIGHTTIME
diphenhydramine hcl tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-958 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) D&C RED NO. 30 (UNII: 2S42T2808B) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) STEARIC ACID (UNII: 4ELV7Z65AP) SUCRALOSE (UNII: 96K6UQ3ZD4) SUCROSE (UNII: C151H8M554) Product Characteristics Color purple Score no score Shape ROUND Size 13mm Flavor GRAPE Imprint Code 44;685 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-958-44 3 in 1 CARTON 07/31/2018 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M010 07/31/2018 Labeler - CVS PHARMACY (062312574) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 pack(69842-958) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(69842-958)