Label: ON TARGET- benzoyl peroxide gel
- NDC Code(s): 66915-437-01
- Packager: CoValence, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 20, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Principal Display Panel
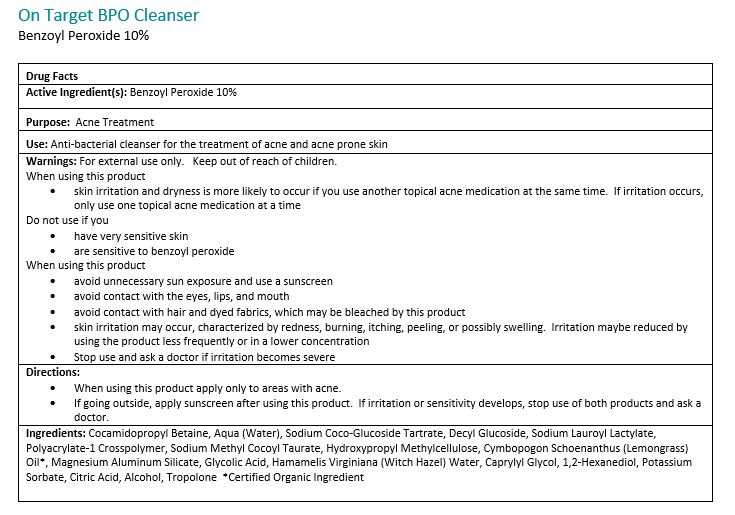
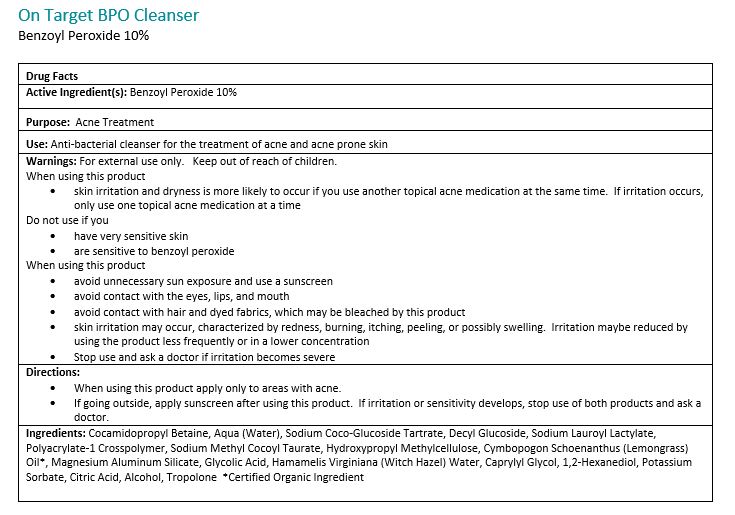
Warnings:
For external use only. Avoide contact with eyes, eyelids, lips and mucous membranes. If accidental contact occurs, rinse with water. Contact with any colored material (including hair and fabric) may result in blaching or discoloration. If exceessive irritation develops, disconintue use and consult your physician.
Directions:
Adults and children 12 years of age or older: Apply to the affected are no more than 3 to 4 times daily.Ingredients:
Cocamidopropyl Betain, Aqua (Water), Sodium Coco-Glucoside Tartrate, Decyl Glucoside, Sodium Lauroyl Lactylate, Polyacrylate-1 Crosspolymer, Sodium Methyl Cocoyl Taurate, Hydroxypropyl Methylcellulose, Cymbopogon Schoenathus (Lemongrass) Oil*, Magnesium Aluminum Silicate, Glycolid Acid, Hamamelis Virginiana (Witch Hazel) Water, Caprylyl Glycol, 1,2-Hexanediol, Potassium Sorbate, Citric Acid, Alcohol, Tropolone
*Certified Organic -
INGREDIENTS AND APPEARANCE
ON TARGET
benzoyl peroxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66915-437 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 0.102 g in 1 g Inactive Ingredients Ingredient Name Strength DECYL GLUCOSIDE (UNII: Z17H97EA6Y) 1 g in 1 g Water (UNII: 059QF0KO0R) 1 g in 1 g SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) 1 g in 1 g SODIUM METHYL COCOYL TAURATE (UNII: JVL98CG53G) 1 g in 1 g WITCH HAZEL (UNII: 101I4J0U34) 1 g in 1 g 1,2-HEXANEDIOL (UNII: TR046Y3K1G) 1 g in 1 g COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) 1 g in 1 g CYMBOPOGON SCHOENANTHUS LEAF (UNII: XF54B1Z2HF) 1 g in 1 g MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) 1 g in 1 g GLYCOLIC ACID (UNII: 0WT12SX38S) 1 g in 1 g CAPRYLYL GLYCOL (UNII: 00YIU5438U) 1 g in 1 g POTASSIUM SORBATE (UNII: 1VPU26JZZ4) 1 g in 1 g CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 1 g in 1 g ALCOHOL (UNII: 3K9958V90M) 1 g in 1 g TROPOLONE (UNII: 7L6DL16P1T) 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66915-437-01 200000 g in 1 DRUM; Type 0: Not a Combination Product 08/18/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 08/18/2011 Labeler - CoValence, Inc. (070653204) Registrant - CoValence, Inc (070653204) Establishment Name Address ID/FEI Business Operations CoValence, Inc. 070653204 manufacture(66915-437)