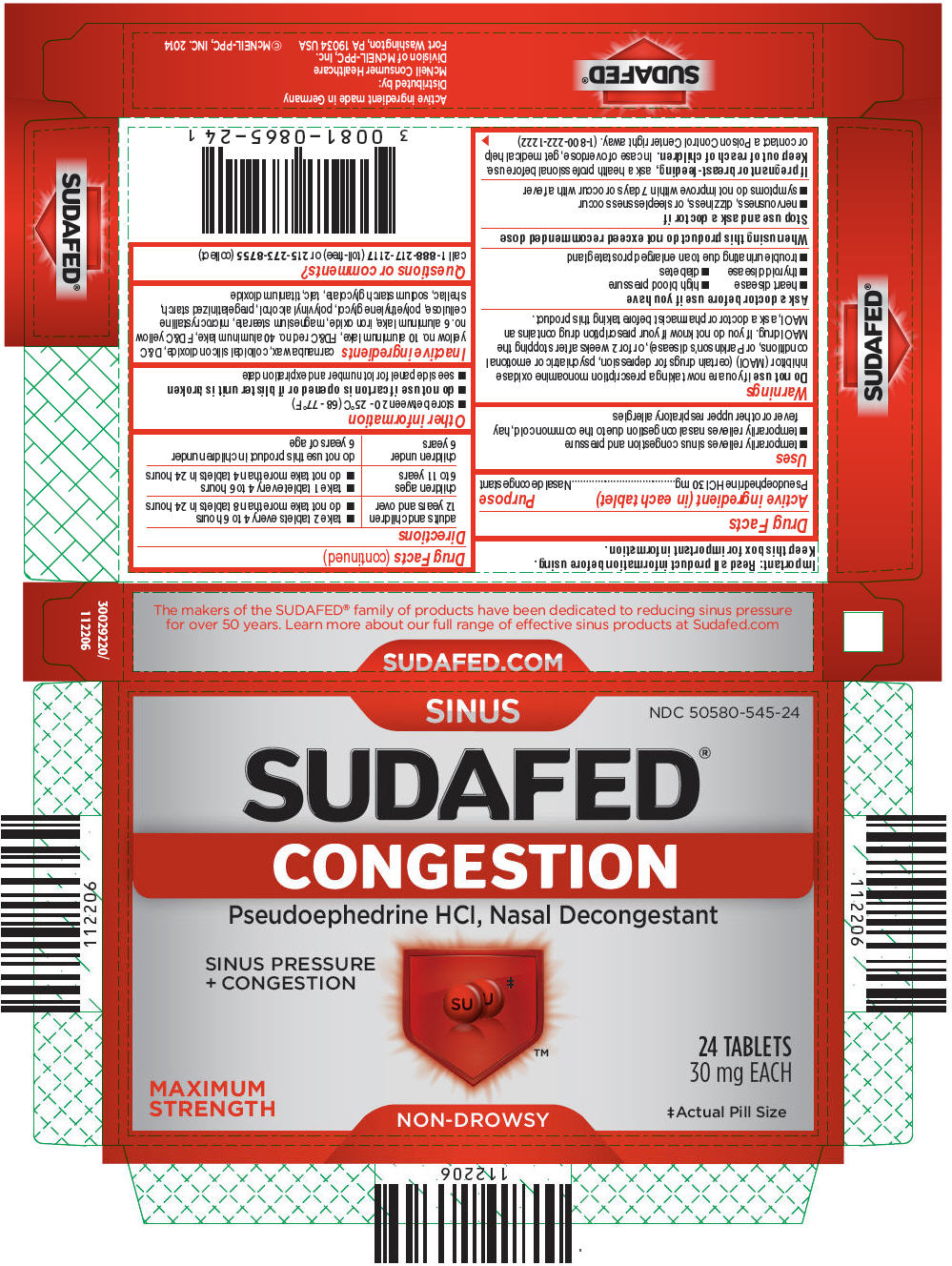
SUDAFED- pseudoephedrine hydrochloride tablet, coated
Johnson & Johnson Consumer Inc., McNeil Consumer Healthcare Division
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (in each tablet)
Pseudoephedrine HCl 30 mg
Purpose
Nasal decongestant
Uses
- temporarily relieves sinus congestion and pressure
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
When using this product do not exceed recommended dose
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- symptoms do not improve within 7 days or occur with a fever
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
| adults and children 12 years and over |
- take 2 tablets every 4 to 6 hours
- do not take more than 8 tablets in 24 hours
|
| children ages 6 to 11 years |
- take 1 tablet every 4 to 6 hours
- do not take more than 4 tablets in 24 hours
|
| children under 6 years | do not use this product in children under 6 years of age |
Other information
- store between 20 - 25°C (68 - 77°F)
-
do not use if carton is opened or if blister unit is broken
- see side panel for lot number and expiration date
Inactive ingredients
carnauba wax, colloidal silicon dioxide, D&C yellow no. 10 aluminum lake, FD&C red no. 40 aluminum lake, FD&C yellow no. 6 aluminum lake, iron oxide, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, pregelatinized starch, shellac, sodium starch glycolate, talc, titanium dioxide
Questions or comments?
call
1-888-217-2117 (toll-free) or
215-273-8755 (collect)
PRINCIPAL DISPLAY PANEL
SINUS
NDC 50580-545-24
SUDAFED®
CONGESTION
Pseudoephedrine HCl, Nasal Decongestant
SINUS PRESSURE
+ CONGESTION
MAXIMUM
STRENGTH
24 TABLETS
30 mg EACH
‡Actual Pill Size
NON-DROWSY