DESQUAM-X WASH- benzoyl peroxide solution
Sun Pharmaceutical Industries, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
WATER BASE
Desquam-X 5 Wash
(5% benzoyl peroxide)
WATER BASE
Desquam-X 10 Wash
(10% benzoyl peroxide)
Rx only
DESCRIPTION
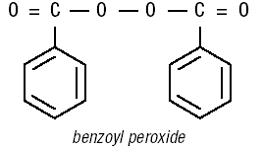
DESQUAM-X 5 (5% benzoyl peroxide) and DESQUAM-X 10 (10% benzoyl peroxide) brand topical therapeutic anti-acne cleansers contains benzoyl peroxide (5% and 10% respectively) in a lathering water base of sodium octoxynol- 3 sulfonate, dioctyl sodium sulfo-succinate, magnesium aluminum silicate, methylcellulose and EDTA.
CLINICAL PHARMACOLOGY
The effectiveness of benzoyl peroxide in the treatment of acne vulgaris is primarily attributable to its antibacterial activity, especially with respect to Propionibacterium acnes, the predominant organism in sebaceous follicles and comedones. 1,2 The antibacterial activity of this compound is presumably due to the release of active or free-radical oxygen capable of oxidizing bacterial proteins. 3 In acne patients treated topically with benzoyl peroxide, resolution of the acne usually coincides with reduction in the levels of P. acnes and free fatty acids (FFA). Mild desquamation is another observed action of topically applied benzoyl peroxide and may also play a role in the drug’s effectiveness in acne. 3 Studies also indicate that topical benzoyl peroxide may exert a se-bostatic effect with a resultant reduction of skin surface lipids. 4,5
Benzoyl peroxide has been shown to be absorbed by the skin, where it is metabolized to benzoic acid and then excreted as benzoate in the urine. 6
INDICATIONS AND USAGE
DESQUAM-X 5 and DESQUAM-X 10 Wash are indicated for the topical treatment of mild to moderate acne. In more severe cases, it may be used as an adjunct in therapeutic regimens including benzoyl peroxide gels, antibiotics, retinoic acid products and sulfur/salicylic acid-containing preparations. The improvement of the treated condition is dependent on the degree and type of acne, the frequency of use of DESQUAM-X WASH and the nature of other therapies employed.
CONTRAINDICATIONS
This product should not be used in patients known to be sensitive to benzoyl peroxide or any other of the listed ingredients.
PRECAUTIONS
General:
Avoid contact with eyes and other mucous membranes. For external use only. In patients known to be sensitive to the following substances, there is a possibility of cross-sensitization: benzoic acid derivatives (including certain topical anesthetics) and cinnamon.
Information for Patients:
This product may bleach colored fabric or hair. Concurent use with PABA-containing sunscreens may result in transient discoloration of the skin.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Based upon considerable evidence, benzoyl peroxide is not considered to be a carcinogen. However, in one study, using mice known to be highly susceptible to cancer, there was evidence for benzoyl peroxide as a tumor promoter. Benzoyl peroxide has been found to be inactive as a mutagen in the Ames Salmonella and other assays, including the mouse dominant lethal assay. This assay is frequently used to assess the effect of substances on spermatogenesis.
Pregnancy (Category C):
Animal reproduction studies have not been conducted with benzoyl peroxide (DESQUAM-X WASH). It is also not known whether benzoyl peroxide can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Benzoyl peroxide should be given to a pregnant woman only if clearly needed.
ADVERSE REACTIONS
Adverse reactions which may be encountered with topical benzoyl peroxide include excessive drying (manifested by marked peeling, erythema and possible edema), and allergic contact sensitization.
Excessive dryness would appear to occur in approximately 2 patients in 50.
Pertinent literature would seem to indicate that allergic sensitization to benzoyl peroxide may occur in 10 to 25 patients in 1,000. 7,8,9 There is one reference that reports an occurrence of sensitization in 5 of 100 patients. 10
OVERDOSAGE
In the event that excessive scaling, erythema or edema occur, the use of this preparation should be discontinued. If the reaction is judged to be due to excessive use and not allergenicity, after symptoms and signs subside, a reduced dosage schedule may be cautiously tried.
To hasten resolution of the adverse effects, emollients, cool compresses and/or topical corticosteroid preparations may be used.
DOSAGE AND ADMINISTRATION
Shake well before use. Wash affected areas once or twice daily, avoiding contact with eyes or mucous membranes. Wet skin areas to be treated prior to administration; apply DESQUAM-X WASH, work to a full lather, rinse thoroughly and pat dry. The amount of drying or peeling may be controlled by modification of dose frequency or drug concentration.
HOW SUPPLIED
Desquam-X Wash (5%): 5 oz. plastic bottle, NDC 10631-284-05.
Desquam-X Wash (10%): 5 oz. plastic bottle, NDC 10631-285-05.
Store at controlled room temperature (59 °-86 °F; 15 °-30 °C).
REFERENCES
- Kligman AM, Leyden JJ, Stewart R: New uses for benzoyl peroxide: A broad-spectrum antimicrobial agent. Int J Derm 16:413-417, 1977.
- Leyden JJ, Stewart R, Kligman AM: Updated invivo methods for evaluating topical antimicrobial agents on human skin. J Invest Dermatol 72:165-170, 1979.
- Fulton JE, Farzad-Bakshandeh A, Bradley S: Studies on the mechanism of action of topical benzoyl peroxide and vitamin-A acid in acne vulgaris. J Cut Path 1:191-200, 1974.
- Fanta D, Jurecka W: Autora-diographic investigation on benzoyl peroxide treated skin. ActaDermatovener 58:361-362, 1978.
- Fanta D, Muller MM: Effect of benzoyl peroxide on skin surface lipids. Dermatologica 158:55-59, 1979.
- Nacht S, Yeung D, Beasley J, Anjo M, Maibach HI: Benzoyl peroxide invitro and invivo skin penetration and metabolic disposition. Presented to Soc for Invest Derm, June 9-14, 1979. Amsterdam.
- Eaglstein WH: Allergic contact dermatitis to benzoyl peroxide. Arch Dermatol 97:527, 1968.
- Pace WE: A benzoyl peroxide-sulfur cream for acne vulgaris. Canad Med Assoc J 93:252-253, 1965.
- Vasarinsh P: Benzoyl peroxide-sulfur lotions in acne vulgaris — a controlled study. Cutis 5(1):65-69, 1969.
- Fanta D: Clinical and experimental studies with benzoyl peroxide in the treatment of acne. Hautzart 29:481- 486, 1978.
| DESQUAM-X
WASH
benzoyl peroxide solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| DESQUAM-X
WASH
benzoyl peroxide solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Sun Pharmaceutical Industries, Inc. (146974886) |