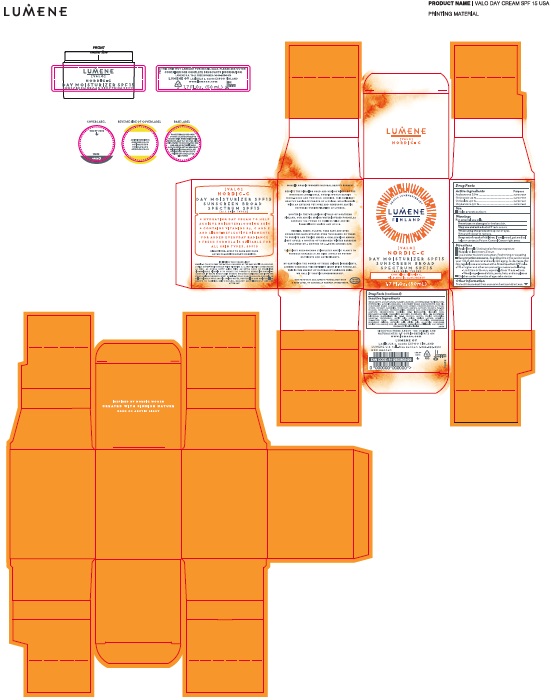
INACTIVE INGREDIENTS:
AQUA (WATER), HEPTYL UNDECYLENATE, CETEARYL ALCOHOL, HYDROGENATED POLYISOBUTENE, PROPANEDIOL, GLYCERIN, SQUALANE, PHENOXYETHANOL, BUTYLENE GLYCOL, CETEARYL GLUCOSIDE, TOCOPHERYL ACETATE, GLYCERYL STEARATE, PEG-100 STEARATE, PANTHENOL, POLYACRYLAMIDE, XANTHAN GUM, ACRYLATES/C1O-30 ALKYL ACRYLATE CROSSPOLYMER, LECITHIN, C13-14 ISOPARAFFIN, (CI 77891) TITANIUM DIOXIDE, DISODIUM EDTA, ETHYLHEXYLGLYCERIN, MAGNESIUM ASCORBYL PHOSPHATE, MICA, HIPPOPHAE RHAMNOIDES (SEA BUCKTHORN) OIL, RUBUS CHAMAEMORUS (CLOUDBERRY) SEED OIL, PEG-8, CAPRYLYL GLYCOL, SODIUM HYDROXIDE, LAURETH-7, TOCOPHEROL, LACTIC ACID, SERINE, SODIUM LACTATE, SORBITOL TEA-LACTATE, UREA, RUBUS CHAMAEMORUS (CLOUDBERRY) SEED EXTRACT, ASCORBYL PALMITATE, SODIUM CHLORIDE, CHONDRUS CRISPUS (CARRAGEENAN), ASCORBIC ACID, CITRIC ACID, ALLANTOIN, HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL, ROSMARINUS OFFICINALIS (ROSEMARY) LEAF EXTRACT, LIMONENE, LINALOOL, BUTYLPHENYL METHYLPROPIONAL, GERANIOL, CITRAL, HEXYL CINNAMAL, CITRONELLOL, PARFUM(FRAGRANCE)