ACETAMINOPHEN- acetaminophen tablet, film coated, extended release
Kroger Company
----------
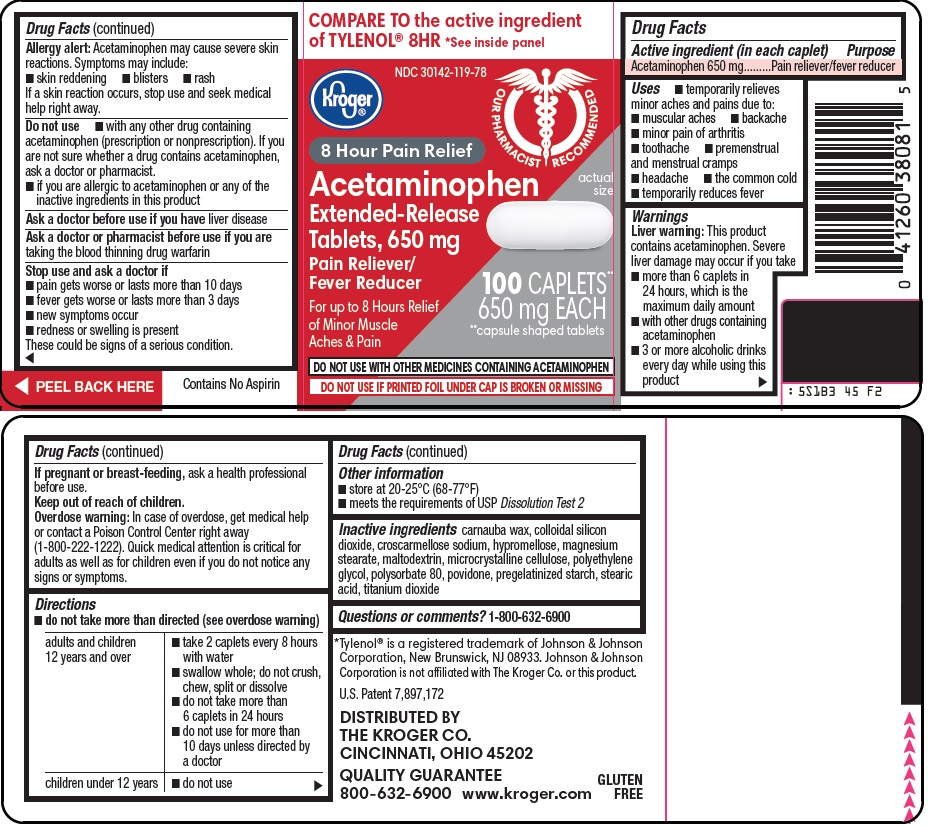
Kroger Co. Acetaminophen Drug Facts
Uses
- •
- temporarily relieves minor aches and pains due to:
- •
- muscular aches
- •
- backache
- •
- minor pain of arthritis
- •
- toothache
- •
- premenstrual and menstrual cramps
- •
- headache
- •
- the common cold
- •
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- •
- more than 6 caplets in 24 hours, which is the maximum daily amount
- •
- with other drugs containing acetaminophen
- •
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- •
- skin reddening
- •
- blisters
- •
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- •
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
Directions
- •
- do not take more than directed (see overdose warning)
|
adults and children 12 years and over |
|
|
children under 12 years |
|
Inactive ingredients
carnauba wax, colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, polysorbate 80, povidone, pregelatinized starch, stearic acid, titanium dioxide
Package/Label Principal Display Panel
COMPARE TO the active ingredient of TYLENOL® 8HR See inside panel
OUR PHARMACIST RECOMMENDED
8 Hour Pain Relief
actual size
Acetaminophen Extended-Release Tablets, 650 mg
Pain Reliever/Fever Reducer
For up to 8 Hours Relief of Minor Muscle Aches & Pain
100 CAPLETS** 650 mg EACH
**capsule-shaped tablets
DO NOT USE WITH OTHER MEDICINES CONTAINING ACETAMINOPHEN
DO NOT USE IF PRINTED FOIL UNDER CAP IS BROKEN OR MISSING
| ACETAMINOPHEN
acetaminophen tablet, film coated, extended release |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Kroger Company (006999528) |