KENZAPUR
- glonoinum, lachesis mutus, natrum muriaticum, natrum sulphuricum, thuja occidentalis liquid
OHM PHARMA INC.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
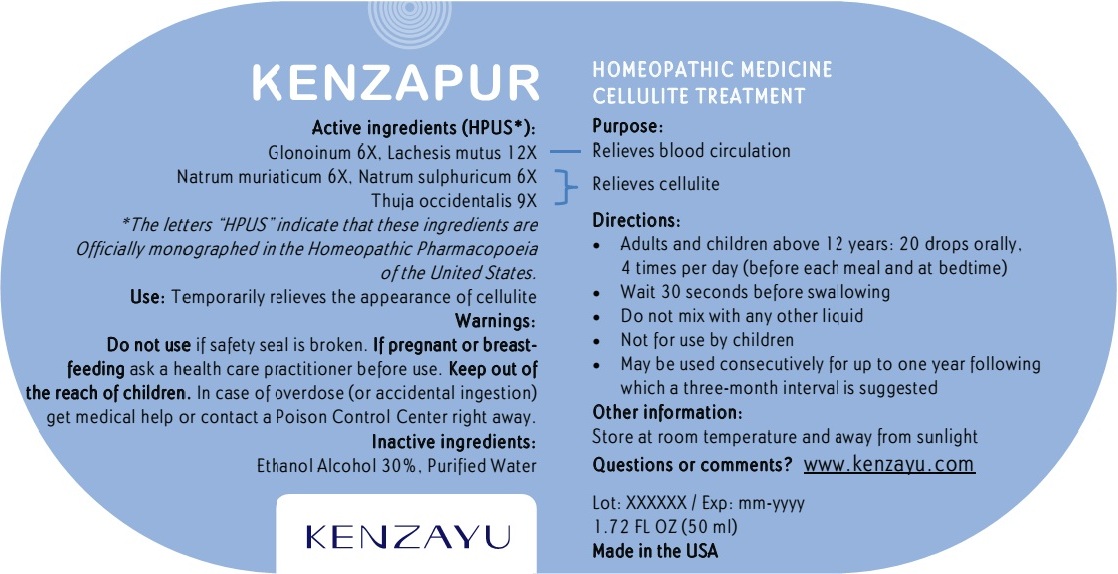
KENZAPUR
| Active ingredients (HPUS*): | Purpose: |
| Glonoinum 6X, Lachesis mutus 12X .............................................................................................. | Relieves blood stasis and poor circulation |
| Natrum muriaticum 6X, Natrum sulphuricum 6X, Thuja Occidentalis 9X ........................................ | Relieves sodium and water retention in tissues |
Warnings:
Do not use if safety seal is broken. If pregnant or breast-feeding ask a health care practitioner before use. Keep out of the reach of children. In case of overdose (or accidental ingestion) get medical help or contact a Poison Control Center right away.
| KENZAPUR
glonoinum, lachesis mutus, natrum muriaticum, natrum sulphuricum, thuja occidentalis liquid |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - OHM PHARMA INC. (030572478) |
| Registrant - OHM PHARMA INC. (030572478) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| OHM PHARMA INC. | 030572478 | manufacture(66096-688) | |
Revised: 12/2017
Document Id: b2c46e96-3f41-4fa5-84c2-5c05b9527246
Set id: d1545ff9-a0d0-4af3-87ef-1466cffec0d2
Version: 2
Effective Time: 20171219
OHM PHARMA INC.