Label: ESOMEPRAZOLE MAGNESIUM capsule, delayed release
-
NDC Code(s):
69238-1050-1,
69238-1050-2,
69238-1050-3,
69238-1050-4, view more69238-1050-5, 69238-1050-8, 69238-1050-9
- Packager: Amneal Pharmaceuticals NY LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
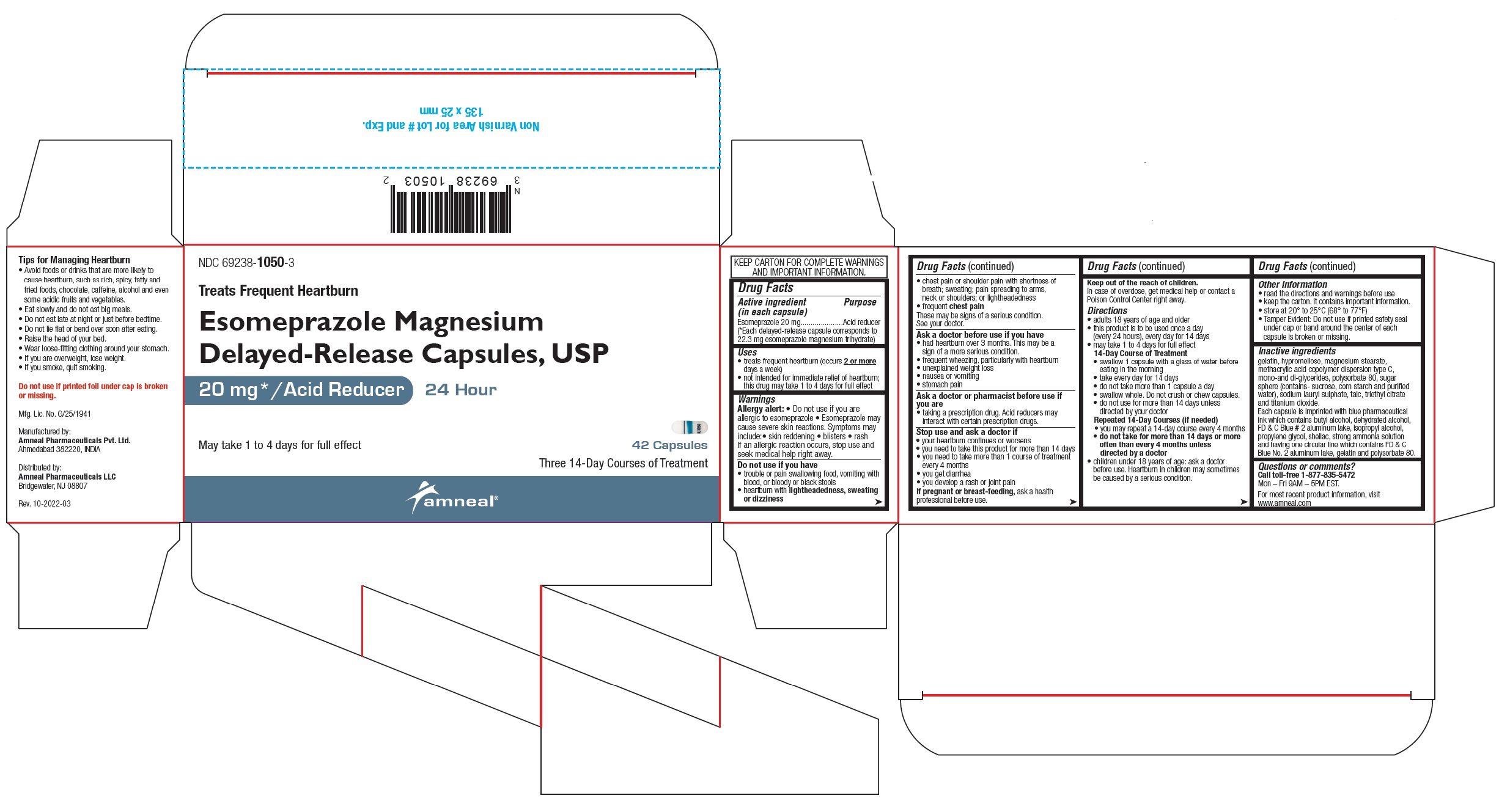
- Drug Facts
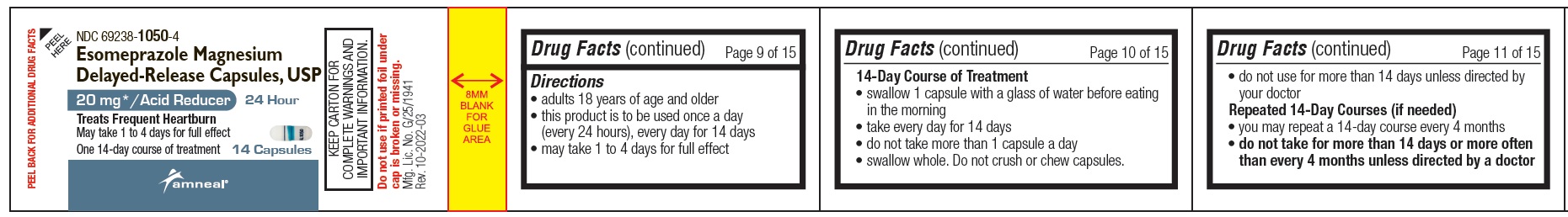
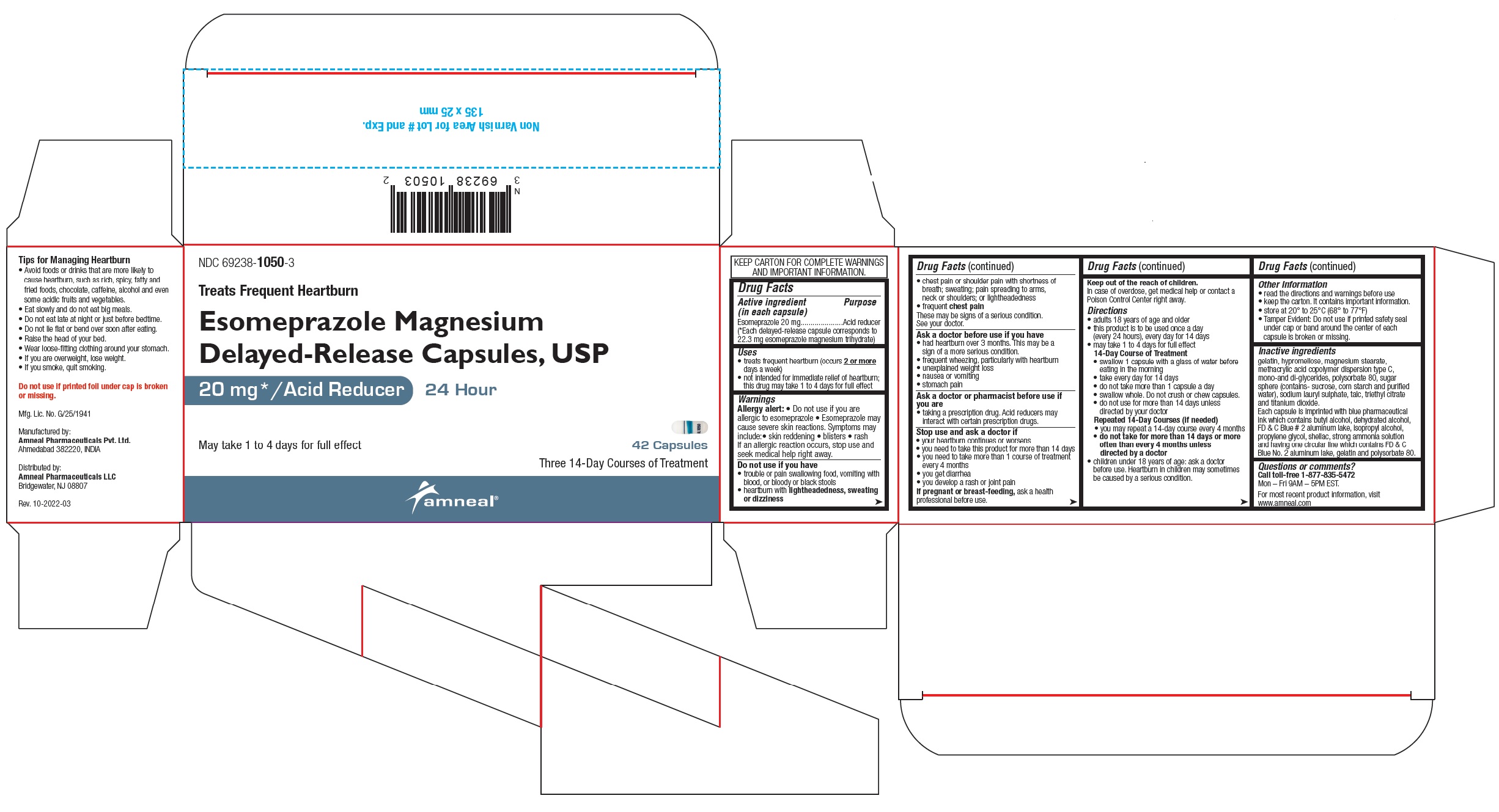
- Uses
-
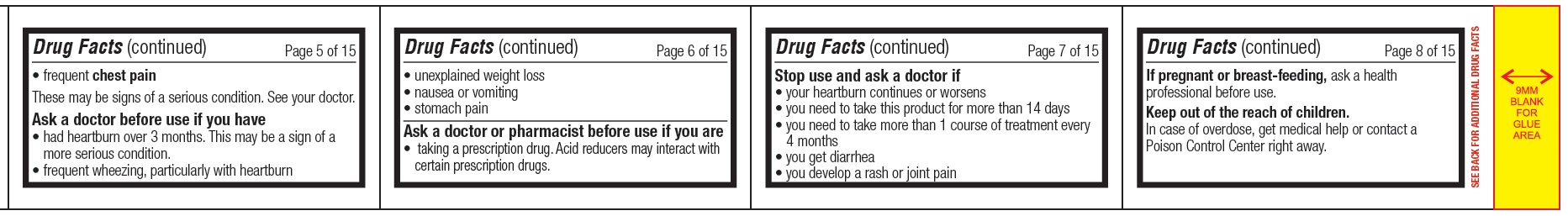
Warnings
Allergy alert:
- Do not use if you are allergic to esomeprazole
- Esomeprazole may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
Do not use if you have
- trouble or pain swallowing food, vomiting with blood, or bloody or black stools
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
Ask a doctor or pharmacist before use if you are
- taking a prescription drug. Acid reducers may interact with certain prescription drugs.
-
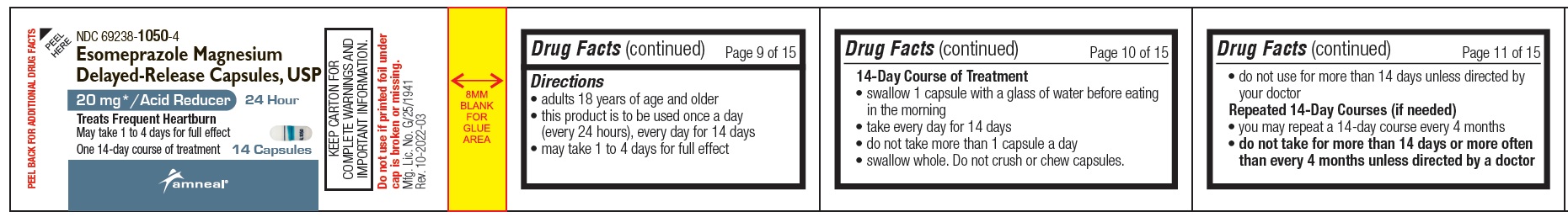
Directions
- adults 18 years of age and older
- this product is to be used once a day (every 24 hours), every day for 14 days
- may take 1 to 4 days for full effect
14-Day Course of Treatment
- swallow 1 capsule with a glass of water before eating in the morning
- take every day for 14 days
- do not take more than 1 capsule a day
- swallow whole. Do not crush or chew capsules.
- do not use for more than 14 days unless directed by your doctor
Repeated 14-Day Courses (if needed)
- you may repeat a 14-day course every 4 months
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
- children under 18 years of age: ask a doctor before use. Heartburn in children may sometimes be caused by a serious condition.
- Other Information
-
Inactive ingredients
gelatin, hypromellose, magnesium stearate, methacrylic acid copolymer dispersion type C, mono-and di-glycerides, polysorbate 80, sugar sphere (contains- sucrose, corn starch and purified water), sodium lauryl sulphate, talc, triethyl citrate and titanium dioxide.
Each capsule is imprinted with blue pharmaceutical ink which contains butyl alcohol, dehydrated alcohol, FD & C Blue # 2 aluminum lake, isopropyl alcohol, propylene glycol, shellac, strong ammonia solution and having one circular line which contains FD & C Blue No. 2 aluminum lake, gelatin and polysorbate 80.
- Questions or comments?
- PURPOSE
-
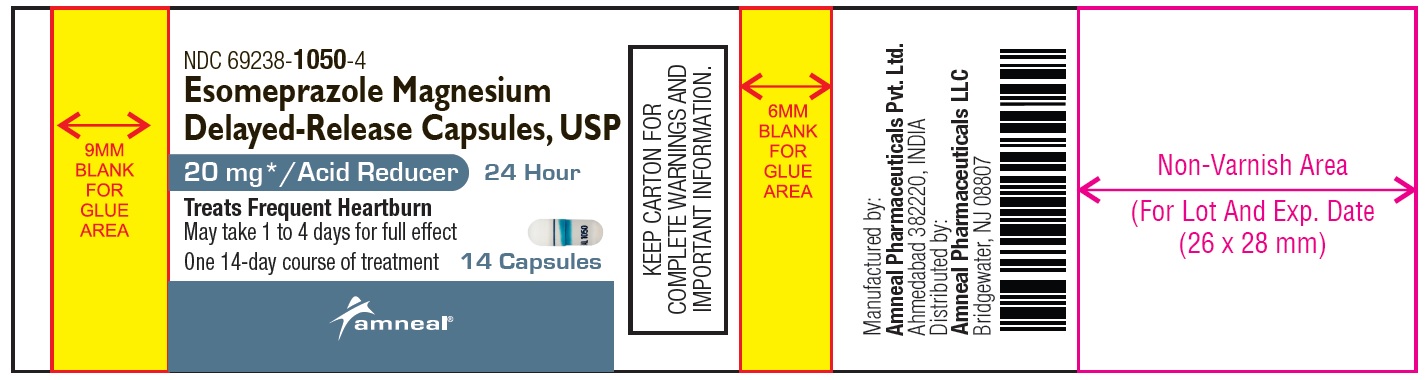
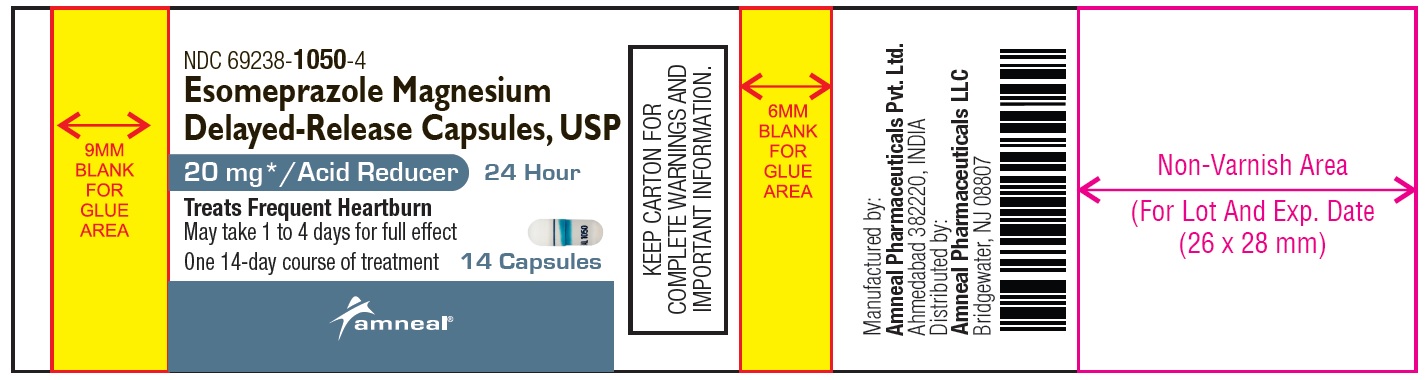
Principal Display Panel
NDC 69238-1050-4
Esomeprazole Magnesium Delayed-Release Capsules, USP/Acid Reducer (OTC)
20 mg
14 Capsules (Container Label)
Amneal Pharmaceuticals LLC


NDC 69238-1050-3
Esomeprazole Magnesium Delayed-Release Capsules, USP /Acid Reducer (OTC)
20 mg
42 Capsules (Carton Pack)
Amneal Pharmaceuticals LLC
-
INGREDIENTS AND APPEARANCE
ESOMEPRAZOLE MAGNESIUM
esomeprazole magnesium capsule, delayed releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69238-1050 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESOMEPRAZOLE MAGNESIUM (UNII: R6DXU4WAY9) (ESOMEPRAZOLE - UNII:N3PA6559FT) ESOMEPRAZOLE 20 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMMONIA (UNII: 5138Q19F1X) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) CAPRYLIC/CAPRIC MONO/DI-GLYCERIDES (UNII: U72Q2I8C85) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) GELATIN (UNII: 2G86QN327L) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) ISOPROPYL ALCOHOL (UNII: ND2M416302) MAGNESIUM STEARATE (UNII: 70097M6I30) METHACRYLIC ACID AND ETHYL ACRYLATE COPOLYMER (UNII: NX76LV5T8J) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STARCH, CORN (UNII: O8232NY3SJ) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) WATER (UNII: 059QF0KO0R) Product Characteristics Color white Score no score Shape CAPSULE Size 14mm Flavor Imprint Code AMNEAL1050 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69238-1050-9 2 in 1 BLISTER PACK 06/05/2019 1 NDC:69238-1050-4 14 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:69238-1050-8 3 in 1 BLISTER PACK 06/05/2019 2 NDC:69238-1050-4 14 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:69238-1050-5 1 in 1 CARTON 06/05/2019 3 NDC:69238-1050-4 14 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:69238-1050-2 2 in 1 CARTON 06/05/2019 4 NDC:69238-1050-4 14 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC:69238-1050-3 3 in 1 CARTON 06/05/2019 5 NDC:69238-1050-4 14 in 1 BOTTLE; Type 0: Not a Combination Product 6 NDC:69238-1050-1 1 in 1 BLISTER PACK 06/05/2019 6 NDC:69238-1050-4 14 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209716 06/05/2019 Labeler - Amneal Pharmaceuticals NY LLC (123797875) Establishment Name Address ID/FEI Business Operations Amneal Pharmaceuticals Private Limited 915076126 analysis(69238-1050) , label(69238-1050) , manufacture(69238-1050) , pack(69238-1050)