ALL DAY RELIEF- naproxen sodium tablet, coated tablet
REMEDYREPACK INC.
----------
NAPROXEN SODIUM- naproxen sodium tablet, coatedMarksans Pharma Limited
OTC - ACTIVE INGREDIENT
Naproxen Sodium Tablets, USP
220 mg (NSAID)*
*nonsteroidal anti-inflammatory drug
INDICATIONS & USAGE
temporarily relieves minor aches and pain due to:
- backache
- headache
- menstrual cramps
- minor pain of arthritis
- muscular aches
- the common cold
- toothache
- temporarily reduces fever
WARNINGS
Allergy alerts: Naproxen sodium may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- asthma (wheezing)
- blisters
- facial swelling
- hives
- rash
- shock
- skin reddening
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have bad stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drug containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product.
- take more or for a longer time than directed.
OTC - DO NOT USE
- if you have ever had an allergic reaction to any other pain reliever / fever reducer
- right before or after heart surgery
OTC - ASK DOCTOR
- the stomach bleeding warning applies to you
- you have a history of stomach problems such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you are taking a diuretic
- you have problems or serious side effects from taking pain relievers or fever reducers
- you have asthma
OTC - WHEN USING
- take with food or milk if stomach upset occurs
- the risk of heart attack or stroke may increase if you use more than directed or for longer than directed
OTC - STOP USE
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have a stomach pain that dose not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- you have difficulty swallowing
- it feels like the pill is sluck in your throat
- redness or swelling is present in the painful area
- any new symptoms appear
OTC - PREGNANCY OR BREAST FEEDING
ask a health professional before use. It is especially important not to use naproxen sodium during the last 3 months of the pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
OTC - KEEP OUT OF REACH OF CHILDREN
In case of accidental overdose, get medical help or contact a poison control center right away.
DOSAGE & ADMINISTRATION
- do not more than directed
- the smallest effective dose should be used
- do not take longer than 10 days, unless directred by a doctor (see Warnings)
- drink a full glass of water with each dose
|
Adults and children 12 years and older:
|
|
|
children under 12 years:
|
|
STORAGE AND HANDLING
- Store at 20-25 oC (68-77 oF). Avoid high humidity and excessive heat above 40 0C (104 0F)
INACTIVE INGREDIENT
Colloidal silicon dioxide, croscarmellose sodium, FD&C blue #2 lake, Hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, titanium dioxide.
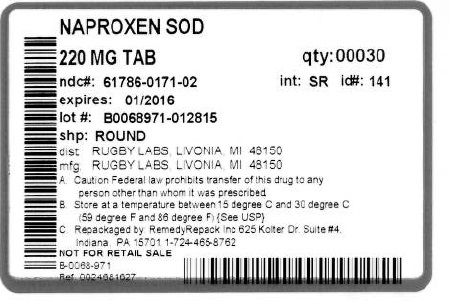
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
DRUG: All Day Relief
GENERIC: Naproxen Sodium Tablet, coated
DOSAGE: TABLET
ADMINSTRATION: ORAL
NDC: 61786-171-02
ACTIVE INGREDIENT(S):
- NAPROXEN SODIUM 220mg in 1
INACTIVE INGREDIENT(S):
- CELLULOSE, MICROCRYSTALLINE
- POLYETHYLENE GLYCOL 1000
- MAGNESIUM STEARATE
- POVIDONE K12
- SILICON DIOXIDE
- CROSCARMELLOSE SODIUM
- FD&C BLUE NO. 2
- HYPROMELLOSES
- TITANIUM DIOXIDE
COLOR: blue
SHAPE: ROUND
SCORE: No score
SIZE: 10 mm
IMPRINT: 141
PACKAGING: 30 in 1 BLISTER PACK
| ALL DAY RELIEF
naproxen sodium tablet, coated tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - REMEDYREPACK INC. (829572556) |