REPASKIN FACIAL, BROAD SPECTRUM SPF 50- octocrylene, titanium dioxide cream
Sesvalia USA LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
REPASKIN FACIAL, BROAD SPECTRUM SPF 50
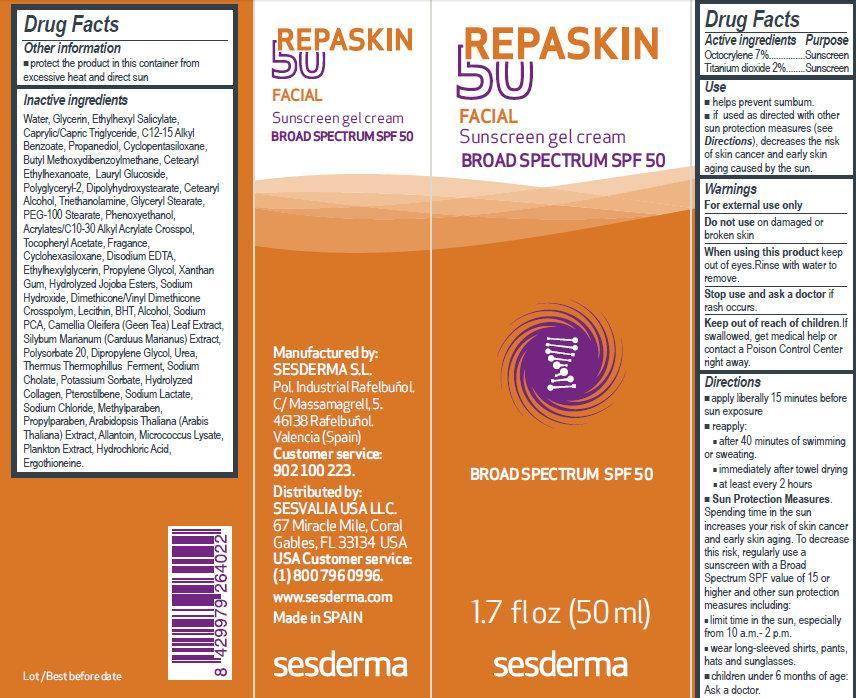
Uses
- helps prevent sumbum.
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- apply liberally 15 minutes before sun exposure
- reapply:
after 40 minutes of swimming or sweating.
immediately after towel drying
at least every 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses.
- children under 6 months of age: Ask a doctor.
Inactive ingredients
Water, Glycerin, Ethylhexyl Salicylate, Caprylic/Capric Triglyceride, C12-15 Alkyl Benzoate, Propanediol, Cyclopentasiloxane, Butyl Methoxydibenzoylmethane, Cetearyl Ethylhexanoate, Lauryl Glucoside, Polyglyceryl-2, Dipolyhydroxystearate, Cetearyl Alcohol, Triethanolamine, Glyceryl Stearate, PEG-100 Stearate,Phenoxyethanol, Acrylates/C10-30 Alkyl Acrylate Crosspol, Tocopheryl Acetate, Fragance, Cyclohexasiloxane, Disodium EDTA, Ethylhexylglycerin, Propylene Glycol, Xanthan Gum, Hydrolyzed Jojoba Esters, Sodium Hydroxide, Dimethicone/Vinyl Dimethicone Crosspolym, Lecithin, BHT, Alcohol, Sodium PCA, Camellia Oleifera (Geen Tea) Leaf Extract, Silybum Marianum (Carduus Marianus) Extract, Silybum Marianum (Carduus Marianus) Extract,Polysorbate 20, Dipropylene Glycol, Urea, Thermus Thermophillus Ferment, Sodium Cholate, Potassium Sorbate, Hydrolyzed Collagen, Pterostilbene, Sodium Lactate, Sodium Chloride, Methylparaben, Propylparaben, Arabidopsis Thaliana (Arabis Thaliana) Extract, Allantoin, Micrococcus Lysate, Plankton Extract, Hydrochloric Acid,Ergothioneine.
Manufactured by: SESDERMA S.L. Pol. Industrial Rafelbuñol. C/ Massamagrell, 5. 46138 Rafelbuñol.Valencia (Spain) Customer service: 902 100 223. Distributed by: SESVALIA USA LLC. 67 Miracle Mile, Coral Gables, FL 33134 USA. USA Customer service: (1) 800 796 0996. www.sesderma.com Made in SPAIN
REPASKIN FACIAL BROAD SPECTRUM SPF 50, 1.7 fl oz (50 ml) (63181-0011-1)
REPASKIN 50
FACIAL
Sunscreen gel cream
BROAD SPECTRUM SPF 50
1.7 fl oz (50 ml)
sesderma
Manufactured by:
SESDERMA S.L. Pol. Industrial Rafelbuñol. C/ Massamagrell, 5. 46138 Rafelbuñol.Valencia (Spain)
Customer service: 902 100 223.
Distributed by:
SESVALIA USA LLC.
67 Miracle Mile, Coral Gables, FL 33134 USA. USA
Customer service: (1) 800 796 0996.
Made in SPAIN
| REPASKIN FACIAL, BROAD SPECTRUM SPF 50
octocrylene, titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Sesvalia USA LLC (061096093) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sesderma S.L. | 633606975 | manufacture(63181-0011) | |