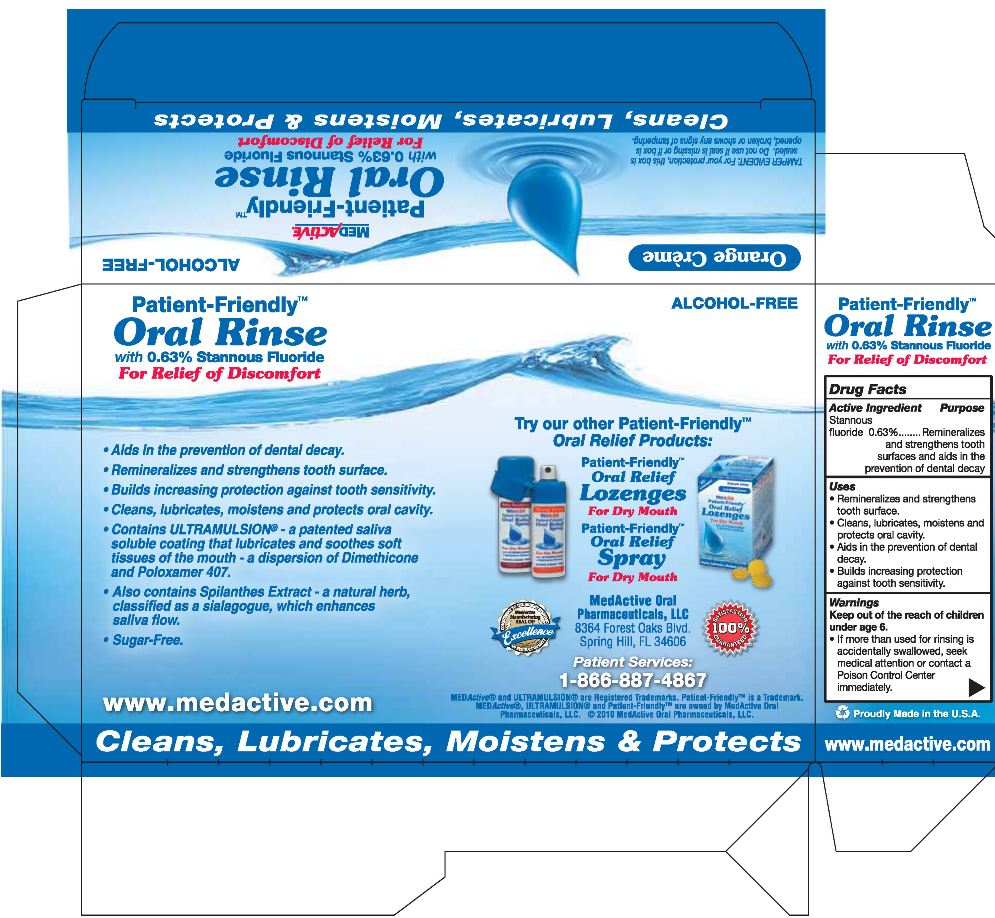
MEDACTIVE PATIENT FRIENDLY- stannous fluoride
Integrate Oral Care, LLC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Stannous Fluoride 0.63%
Purpose
Remineralizes and strengthens tooth surfaces and aids in the prevention of dental decay.
Keep out of the reach of children under age 6.
- If more than used for rinsing is accidentally swallowed, seek medical attention or contact a Poison Control Center immediately.
Uses
- Remineralizes and strengthens tooth surface.
- Cleans, lubricates, moistens and protects oral cavity.
- Aids in the prevention of dental decay.
- Builds increasing protection against tooth sensitivity.
Other information
- This product may produce surface staining of the teeth. Adequate toothbrushing may prevent these stains which are not harmful or permanent and may be removed by your dentist.
- Do not refrigerate. Store at room temperature.
- See box for expiration date.
Directions
- Use immediately after preparing the rinse.
For adults and children 6 years of age and older:
Place both thumbs on
Blue Chamber No. 1 and Press down firmly to squeeze fluoride into
Chamber No. 2.
- Gently Shake.
- Snap Tab to Open by bending at line on cap. Dispense half of mixture into mouth and Rinse for 30 seconds. Spit.
- Gargle with remainder of mixture for 30 seconds. Spit.
Avoid any food or drinks for at least 15 minutes after use.
For children under 6 years of age: consult a dentist or doctor.
Inactive ingredients
Water, Sorbitol, Glycerin, ULTRAMULSION®, Potassium Sorbate, EDTA, Sucralose, Flavor
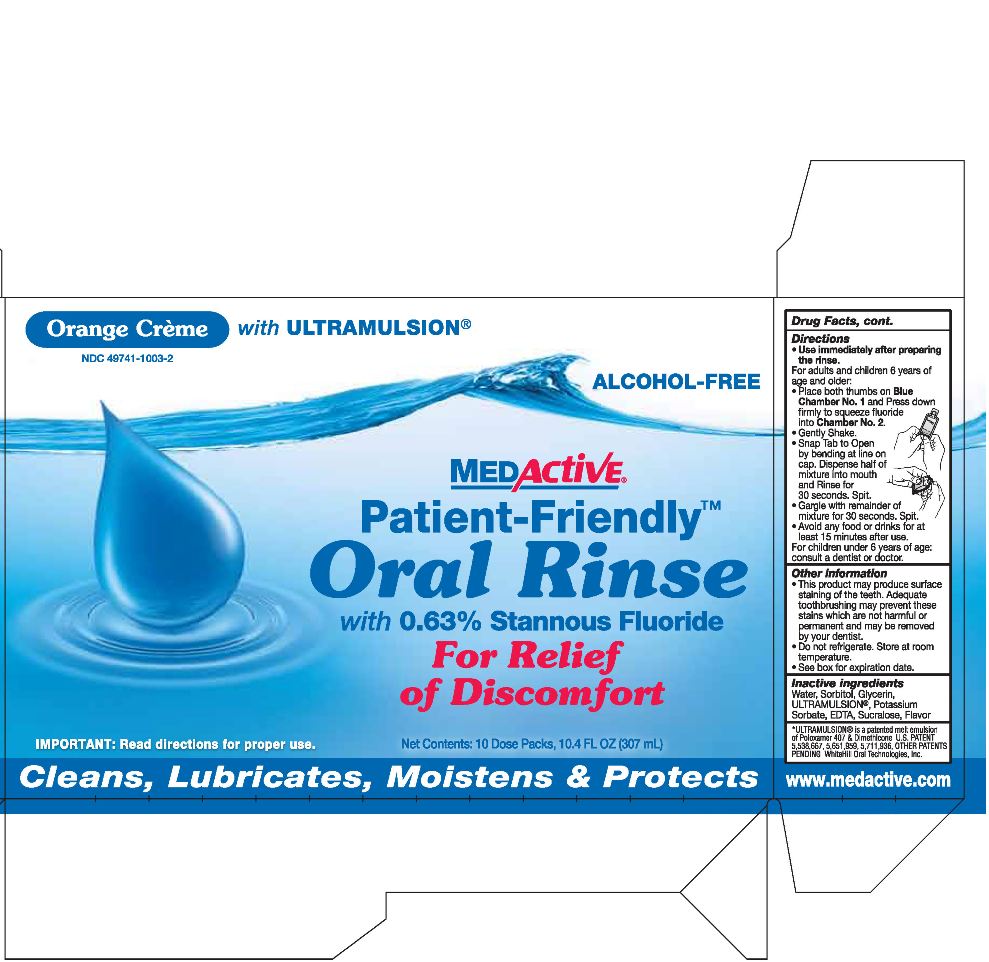
Orange Crème with
ULTRAMULSION®
ALCOHOL-FREE
MEDActive®
Patient-Friendly®
Oral Rinse
with 0.63% Stannous Fluoride
For Relief of Discomfort
IMPORTANT: Read directions for proper use.
Net Contents: 10 Dose Packs, 10.4 FL OZ (307ml)
Integrate Oral Care, LLC.

