LIDOCAINE HYDROCHLORIDE- lidocaine hydrochloride injection
Amphastar Pharmaceuticals, Inc.
----------
LIDOCAINE HYDROCHLORIDE
INJECTION, USP
{FOR VENTRICULAR ARRHYTHMIAS}
DESCRIPTION
Lidocaine Hydrochloride Injection USP, is a sterile, aqueous solution of lidocaine, an antiarrhythmic agent, prepared with the aid of hydrochloric acid. It is intended for intravenous administration by either direct injection or continuous infusion.
Lidocaine hydrochloride is designated 2-(Diethylamino)-2', 6'-acetoxylidide monohydrochloride and is represented by the following structural formula:
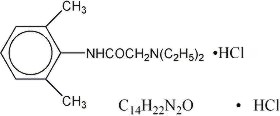
Composition of available solutions: M.W. 270.80
Concentration Lidocaine HCl
(mg per mL) Sodium Chloride
(mg per mL)
to adjust tonicity
For Direct Injection 2% 20 6
The medication and fluid pathway of these disposable syringes are sterile and nonpyrogenic in the original, unopened package with component caps in place. These dosage forms do not contain preservatives; once the unit is assembled and used, any remaining portion of the solution must be discarded with the entire unit.
pH of the above solution adjusted with sodium hydroxide and/or hydrochloric acid to finished product pH limits between 5 and 7.
CLINICAL PHARMACOLOGY
Mechanisms of action and electrophysiology
Studies of the effects of therapeutic concentrations of lidocaine on the electrophysiological properties of mammalian Purkinje fibers have shown that lidocaine attenuates phase 4 diastolic depolarization, decreases automaticity and causes a decrease or no change in excitability and membrane responsiveness. Action potential duration and effective refractory period of Purkinje fibers are decreased, while the ratio of effective refractory period to action potential duration is increased. Action potential duration and effective refractory period of ventricular muscle are also decreased. Effective refractory period of the AV node may increase, decrease or remain unchanged, and atrial effective refractory period is unchanged. Lidocaine raises the ventricular fibrillation threshold. No significant interactions between lidocaine and the autonomic nervous system have been described and consequently lidocaine has little or no effect on autonomic tone.
Clinical electrophysiological studies with lidocaine have demonstrated no change in sinus node recovery time or sinoatrial conduction time. AV nodal conduction time is unchanged or shortened, and His-Purkinje conduction time is unchanged.
Hemodynamics
At therapeutic doses, lidocaine has minimal hemodynamic effects in normal subjects and in patients with heart disease. Lidocaine has been shown to cause no, or minimal, decrease in ventricular contractility, cardiac output, arterial pressure or heart rate.
Pharmacokinetics and metabolism
Lidocaine is rapidly metabolized by the liver, and less than 10% of a dose is excreted unchanged in the urine. Oxidative N dealkylation, a major pathway of metabolism, results in the metabolites monoethylglycinexylidide and glycinexylidide. The pharmacological/toxicological activities of these metabolites are similar to, but less potent than, lidocaine. The primary metabolite in urine is a conjugate of 4-hydroxy-2,6,-dimethylaniline.
The elimination half-life of lidocaine following an intravenous bolus injection is typically 1.5 to 2 hours. There are data that indicate that the half-life may be 3 hours or longer following infusions of greater than 24 hours.
Because of the rapid rate at which lidocaine is metabolized, any condition that alters liver function, including changes in liver blood flow, which could result from severe congestive heart failure in shock, may alter lidocaine kinetics. The half-life may be two-fold or more, greater in patients with liver dysfunction. Renal dysfunction does not affect lidocaine kinetics, but may increase the accumulation of metabolites.
Therapeutic effects of lidocaine are generally associated with plasma levels at 6 to 25 µmole/L (1.5 to 6 mcg free base per mL). The blood to plasma distribution ratio is approximately 0.84. Objective adverse manifestations become increasingly apparent with increasing plasma levels above 6 mcg free base per mL.
The plasma protein binding of lidocaine is dependent on drug concentration, and the fraction bound decreases with increasing concentration. At concentrations of 1 to 4 mcg free base per mL, 60 to 80 percent of lidocaine is protein bound. In addition to lidocaine concentration, the binding is dependent on the plasma concentration of the α-1-acid glycoprotein.
Lidocaine readily crosses the placental and blood-brain barriers. Dialysis has negligible effects on the kinetics of lidocaine.
INDICATIONS AND USAGE
Lidocaine hydrochloride administered intravenously, is specifically indicated in the acute management of ventricular arrhythmias such as those occurring in relation to acute myocardial infarction, or during cardiac manipulation, such as cardiac surgery.
CONTRAINDICATIONS
Lidocaine hydrochloride is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type. Lidocaine hydrochloride should not be used in patients with Stokes-Adams syndrome. Wolff-Parkinson-White syndrome, or with severe degrees of sinoatrial, atrioventricular, or intraventricular block in the absence of an artificial pacemaker.
WARNINGS
IN ORDER TO MANAGE POSSIBLE ADVERSE REACTIONS, RESUSCITATIVE EQUIPMENT, OXYGEN AND OTHER RESUSCITATIVE DRUGS SHOULD BE IMMEDIATELY AVAILABLE WHEN LIDOCAINE HYDROCHLORIDE INJECTION IS USED.
Systemic toxicity may result in manifestations of central nervous system depression (sedation) or irritability (twitching), which may progress to frank convulsions accompanied by respiratory depression and/or arrest. Early recognition of premonitory signs, assurance of adequate oxygenation and, where necessary, establishment of artificial airway with ventilatory support are essential to management of this problem. Should convulsions persist despite ventilatory therapy with oxygen, small increments of anticonvulsant drugs may be used intravenously. Examples of such agents include benzodiazepines (e.g., diazepam), ultra short-acting barbiturates (e.g., thiopental or thiamylal), or a short-acting barbiturate (e.g., pentobarbital or secobarbital). If the patient is under anesthesia, a short-acting muscle relaxant (e.g., succinylcholine) may be used. Longer acting drugs should be used only when recurrent convulsions are evidenced.
Should circulatory depression occur, vasopressors may be used.
Constant electrocardiographic monitoring is essential to the proper administration of lidocaine hydrochloride. Signs of excessive depression of cardiac electrical activity such as sinus node dysfunction, prolongation of the P-R interval and QRS complex or the appearance or aggravation of arrhythmias, should be followed by flow adjustment and, if necessary, prompt cessation of the intravenous infusion of this agent. Occasionally, acceleration of ventricular rate may occur when lidocaine hydrochloride is administered to patients with atrial flutter or fibrillation.
PRECAUTIONS
1. General
Caution should be employed in the use of lidocaine hydrochloride in patients with severe liver or kidney disease because accumulation of the drug or metabolites may occur.
Lidocaine hydrochloride should be used with caution in the treatment of patients with hypovolemia, severe congestive heart failure, shock, and all forms of heart block. In patients with sinus bradycardia or incomplete heart block, the administration of lidocaine hydrochloride intravenously for the elimination of ventricular ectopic beats, without prior acceleration in heart rate (e.g., by atropine, isoproterenol or electric pacing), may promote more frequent and serious ventricular arrhythmias or complete heart block (see CONTRAINDICATIONS).
Dosage should be reduced for children and for debilitated and/or elderly patients, commensurate with their age and physical status.
The safety of amide local anesthetic agents in patients with genetic predisposition to malignant hyperthermia has not been fully assessed; therefore, lidocaine should be used with caution in such patients.
In hospital environments where drugs known to be triggering agents for malignant hyperthermia (fulminant hypermetabolism) are administered, it is suggested that a standard protocol for management should be available.
It is not known whether lidocaine may trigger this reaction; however, large doses resulting in significant plasma concentrations, as may be achieved by intravenous infusion, pose potential risk to these individuals. Recognition of early unexplained signs of tachycardia, tachypnea, labile blood pressure and metabolic acidosis may precede temperature elevation. Successful outcome is dependent on early diagnosis, prompt discontinuance of the triggering agent and institution of treatment including oxygen therapy, supportive measures and dantrolene (for details see dantrolent package insert).
2. Patient information
The patients should be advised of the possible occurrence of the experiences listed under ADVERSE REACTIONS.
4. Drug interactions
Lidocaine hydrochloride should be used with caution in patients with digitalis toxicity accompanied by atrioventricular block. Concomitant use of beta-blocking agents may reduce hepatic blood flow and thereby reduce lidocaine clearance.
Lidocaine and tocainide are pharmacologically similar. The concomitant use of these two agents may cause an increased incidence of adverse reactions, including central nervous system adverse reactions such as seizure.
5. Carcinogenesis, mutagenesis, impairment of fertility
Long term studies in animals to evaluate the carcinogenic and mutagenic potential or the effect on fertility of lidocaine hydrochloride have not been conducted.
6. Pregnancy
Teratogenic effects
Pregnancy Category B
Reproduction studies have been performed in rats at doses up to 6.6 times the maximum human doses and have revealed no significant findings. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predicted of human response, this drug should be used during pregnancy only if clearly needed.
7. Labor and delivery
The effects of lidocaine hydrochloride on the mother and the fetus, when used in the management of cardiac arrhythmias during labor and delivery, are not known. Lidocaine readily crosses the placental barrier.
8. Nursing mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when lidocaine is administered to a nursing woman.
9. Pediatric use
Safety and effectiveness in children have not been established by controlled clinical studies (See DOSAGE AND ADMINISTRATION).
ADVERSE REACTIONS
Adverse experiences following the administration of lidocaine are similar in nature to those observed with other amide local anesthetic agents. Adverse experiences may result from high plasma levels caused by excessive dosage or may result from a hypersensitivity, idiosyncrasy or diminished tolerance on the part of the patient. Serious adverse experiences are generally systemic in nature. The following types are those most commonly reported. The adverse experiences under Central Nervous System and Cardiovascular System are listed, in general, in a progression from mild to severe.
- Central Nervous System: CNS reactions are excitatory and/or depressant, and may be characterized by lightheadedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, respiratory depression and arrest. The excitatory reactions may be very brief or may not occur at all, in which case, the first manifestation of toxicity may be drowsiness, merging into unconsciousness and respiratory arrest.
- Cardiovascular System: Cardiovascular reactions are usually depressant in nature and are characterized by bradycardia, hypotension, and cardiovascular collapse, which may lead to cardiac arrest.
- Allergic reactions as a result of sensitivity to lidocaine are extremely rare and, if they occur, should be managed by conventional means.
DRUG ABUSE AND DEPENDENCE
Although specific studies have not been conducted, lidocaine hydrochloride has been used clinically without evidence of abuse of this drug or of physiological or physical dependence as a result of its use.
OVERDOSAGE
Overdosage of lidocaine hydrochloride usually results in signs of central nervous system or cardiovascular toxicity. See ADVERSE REACTIONS.
Should convulsions or signs of respiratory depression and arrest develop, the patency of the airway and adequacy of ventilation must be assured immediately. Should convulsions persist despite ventilatory therapy with oxygen, small increments of anticonvulsive agents may be given intravenously. Examples of such agents include a benzodiazepine(e.g., diazepam), an ultrashort-acting barbiturate (e.g., thiopental or thiamylal), or a short-acting barbiturate (e.g., pentobarbital or secobarbital). If the patient is under general anesthesia, a short-acting muscle relaxant (e.g., succinylcholine) may be administered.
Should circulatory depression occur, vasopressors may be used. Should cardiac arrest occur, standard CPR procedures should be instituted.
Dialysis is of negligible value in the treatment of acute overdosage from lidocaine hydrochloride.
DOSAGE AND ADMINISTRATION
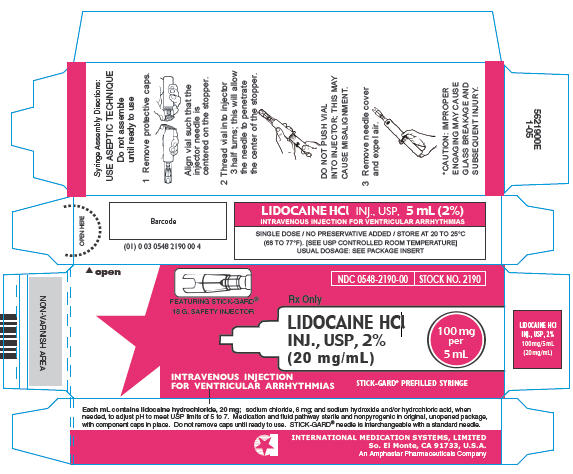
Please note that the optional STICK-GARD® Safety Needle, featured with stock number 2190 is interchangeable with a standard needle.
Adults
Single Direct Intravenous Injection (bolus)
ONLY THE 50 AND 100 MG DOSAGE SIZES should be used for direct intravenous injection. The usual dose is 50 to 100 mg of lidocaine hydrochloride (0.70 to 1.4 mg/kg; 0.32 to 0.63 mg/lb) administered intravenously under ECG monitoring. This dose may be administered at the rate of approximately 25 to 50 mg/min (0.35 to 0.70 mg/kg/min; 0.16 to 0.32 mg/lb/min). Sufficient time should be allowed to enable a slow circulation to carry the drug to the site of action. If the initial injection of 50 to 100 mg does not produce a desired response, a second dose may be injected after 5 minutes. (See illustrated instructions for use.) NO MORE THAN 200 TO 300 MG OF LIDOCAINE HYDROCHLORIDE SHOULD BE ADMINISTERED DURING A ONE HOUR PERIOD.
Continuous Intravenous Infusion
Following bolus administration, intravenous infusions of lidocaine hydrochloride may be initiated at the rate of 1 to 4 mg/min of lidocaine hydrochloride (0.014 to 0.057 mg/kg/min; 0.006 to 0.026 mg/lb/min). The rate of intravenous infusions should be reassessed as soon as the patient's basic cardiac rhythm appears to be stable or at the earliest signs of toxicity. It should rarely be necessary to continue intravenous infusions of lidocaine for prolonged periods.
Solutions for intravenous infusion may be prepared by the addition of one gram (or two grams) of lidocaine hydrochloride to one liter of 5% dextrose in water using aseptic technique. Approximately a 0.1% (or 0.2% ) solution will result from this procedure; that is, each milliliter will contain approximately 1 (or 2) mg of lidocaine hydrochloride. In those cases in which fluid restriction is medically appropriate, a more concentrated solution may be prepared.
Lidocaine hydrochloride injection has been found to be chemically stable for 24 hours after dilution in 5% dextrose in water. However, as with all intravenous admixtures, dilution of the solution should be made just prior to its administration.
It is very important that after adding lidocaine hydrochloride, or any other medication, to an I.V. container, the contents be thoroughly mixed before beginning the infusion.
When administering by continuous I.V. infusion, it is advisable to use a precision volume control I.V. set.
Pediatric
Controlled clinical studies in the pediatric population to establish dosing schedules have not been conducted. The American Heart Association's Standards and Guidelines recommends a bolus dose of 1 mg/kg, and an infusion rate of between 20 to 50 mcg/kg/min for prolonged therapy. When drug clearance is reduced, as in patients with shock, congestive heart failure or cardiac arrest, the infusion rate should not exceed 20 mcg/kg/min.
HOW SUPPLIED
Lidocaine Hydrochloride Injection, USP
For Direct Intravenous Injection
In unit of use packages containing one single dose vial and a MIN-I-JET® vial injector. Boxes of 25 units.
| Concentration | Stock No. | NDC No. | Size |
|---|---|---|---|
| 2% | 1190 | 0548-1190-00 | 5 mL (100 mg) |
In unit of use packages containing a single dose vial and a disposable Luer-Lock syringe featuring optional STICK-GARD® Safety Needle. Boxes of 25.
| Concentration | Stock No. | NDC No. | Size |
|---|---|---|---|
| 2% | 2190 | 0548-2190-00 | 5 mL (100 mg) |
Manufactured under U.S. Pat. No. 4,834,716, reissue No. 33,617 STICK-GARD® Safety Needle.
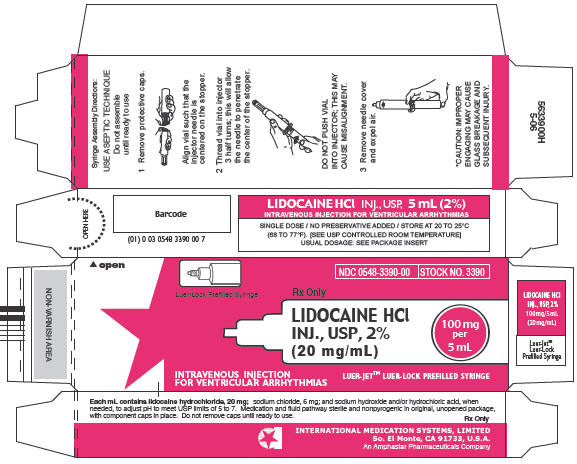
In unit-use packages containing a Luer-Jet™ Luer-Lock Prefilled Syringe. Shrink Wrapped Packages of 10.
| Concentration | Stock No. | NDC No. | Size |
|---|---|---|---|
| 2% | 3390 | 0548-3390-00 | 5 mL (100 mg) |
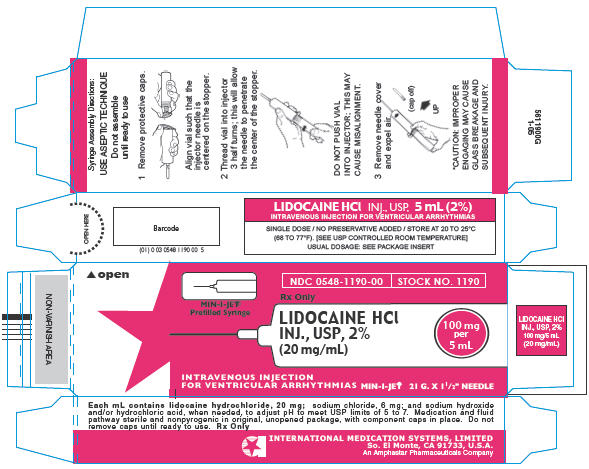
Syringe Assembly Directions
The MIN-I-JET® Syringe with needle, illustrated below, is the basic unit upon which all the other syringe systems are built; slight adaptations and / or additional auxiliary parts create the other syringe systems. Assembly directions remain essentially the same.
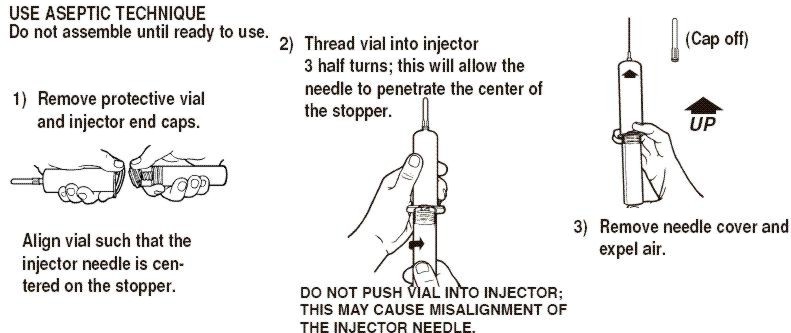
*CAUTION: IMPROPER ENGAGING MAY CAUSE GLASS BREAKAGE AND SUBSEQUENT INJURY.
INTERNATIONAL MEDICATION SYSTEMS, LIMITED
SO. EL MONTE, CA 91733, U.S.A.
An Amphastar Pharmaceuticals Company
REV. 1-08
6911900N
PRINCIPAL DISPLAY PANEL - MIN-I-JET® Carton
NDC 0548-1190-00
STOCK NO. 1190
Rx Only
MIN-I-JET®
Prefilled Syringe
LIDOCAINE HCl
INJ., USP, 2%
(20 mg/mL)
100 mg
per
5 mL
INTRAVENOUS INJECTION
FOR VENTRICULAR ARRHYTHMIAS MIN-I-JET® 21 G. X 1½" NEEDLE
| LIDOCAINE HYDROCHLORIDE
lidocaine hydrochloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LIDOCAINE HYDROCHLORIDE
lidocaine hydrochloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LIDOCAINE HYDROCHLORIDE
lidocaine hydrochloride injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Amphastar Pharmaceuticals, Inc. (024736733) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Amphastar Pharmaceuticals, Inc. | 024736733 | analysis(0548-1190, 0548-2190, 0548-3390) , manufacture(0548-1190, 0548-2190, 0548-3390) , label(0548-1190, 0548-2190, 0548-3390) | |