SECURA EPC SKIN CARE STARTER KIT- zinc oxide,benzethonium chloride
Smith & Nephew, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Secura EPC Skin Care Starter Kit
DRUG FACTS - SECURA EPC SKIN CARE STARTER KIT
Refer to individual product labeling for detailed instructions.
DRUG FACTS - SECURA EXTRA PROTECTIVE CREAM
USES
- Helps treat and prevent diaper dermatitis
- Protects minor skin irritation associated with diaper dermatitis and helps seal out wetness
DIRECTIONS
- change wet and soiled diapers, garments and linens promptly
- cleanse the affected area and allow to dry
- apply cream liberally as often as necessary with each diaper change; especially at bedtime or anytime when exposure to soiled diapers, garments, linens, feces, or urine may be prolonged
DRUG FACTS - SECURA MOISTURIZING CLEANSER
USES
- Antimicrobial skin cleanser for the perineum or body
- Aids in the removal of urine and feces, or other foreign material
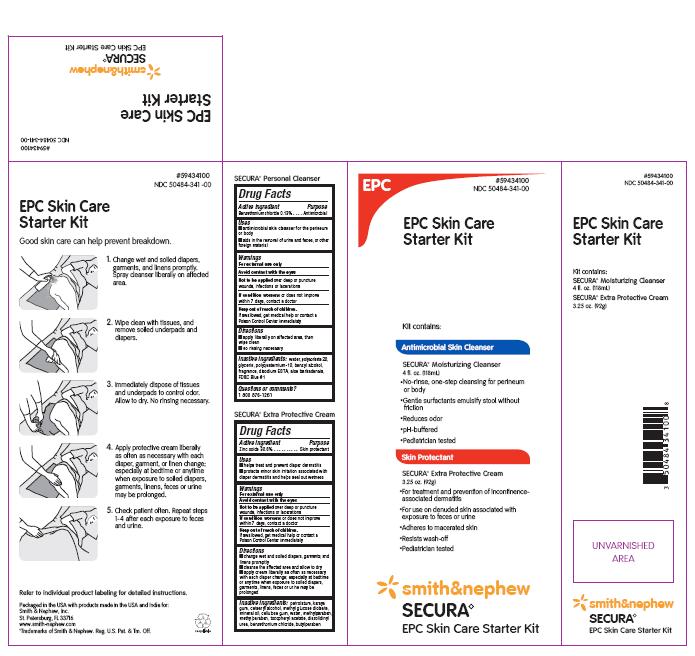
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL- SECURA EPC SKIN CARE STARTER KIT
#59434100
NDC 50484-341-00
EPC Skin Care Starter Kit
Antimicrobial Skin Cleanser
Secura Moisturizing Cleanser 4 fl. oz. (118mL)
- Provides one-step no-rinse cleansing for perineum and body; saves time
- Pediatrician-tested
Skin Protectant
Secura Extra Protective Cream 3.25 oz. (92g)
- Helps treat and prevent diaper dermatitis
- Contains Karaya to adhere to macerated skin
Packaged in the USA with products made in USA and India for:
Smith & Nephew, Inc.
St. Petersburg, FL 33716
www.smith-nephew.com
Trademarks of Smith & Nephew. Reg. U.S. Pat. & Tm. Off.
Smith&Nephew
Secura◊
EPC Skin Care Starter Kit
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - SECURA EXTRA PROTECTIVE CREAM - TUBE (92g)
Item #59432400
NDC 50484-324-00
Extra Protective Cream
Skin Protectant
- For treatment and prevention of incontinence-associated dermatitis
- For use on denuded skin associated with exposure to feces or urine
- Adheres to macerated skin
- Resists wash-off
- Pediatrician tested
Smith & Nephew
Secura◊
Extra Protective Cream
Made in India for:
Smith & Nephew, Inc., St. Petersburg, FL 33716
www.smith-nephew.com
Trademark of Smith & Nephew Reg. U.S. Pat. & Tm. Off.

PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - SECURA MOISTURIZING CLEANSER - BOTTLE (118mL)
Item #59430800
NDC 50484-308-00
Moisturizing Cleanser
Antimicrobial Skin Cleanser
- No-rinse, one-step cleansing for perineum or body
- Gentle surfactants emulsify stool without friction
- Reduces Odor
- pH-balanced
- Contains Aloe and Glycerin to condition, soothe and moisturize skin
- Pediatrician-tested
Smith & Nephew
Secura◊
Moisturizing Cleanser
Made for:
Smith & Nephew, Inc., St. Petersburg, FL
33773
www.smith-nephew.com
Trademark of Smith & Nephew
Reg. U.S. Pat. & Tm. Off.

| SECURA EPC SKIN CARE STARTER KIT
zinc oxide,benzethonium chloride kit |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Smith & Nephew, Inc. (827731451) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Swiss-American CDMO, LLC | 080170933 | MANUFACTURE(50484-324, 50484-341) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ENCUBE ETHICALS PVT LTD | 915834105 | MANUFACTURE(50484-341) | |