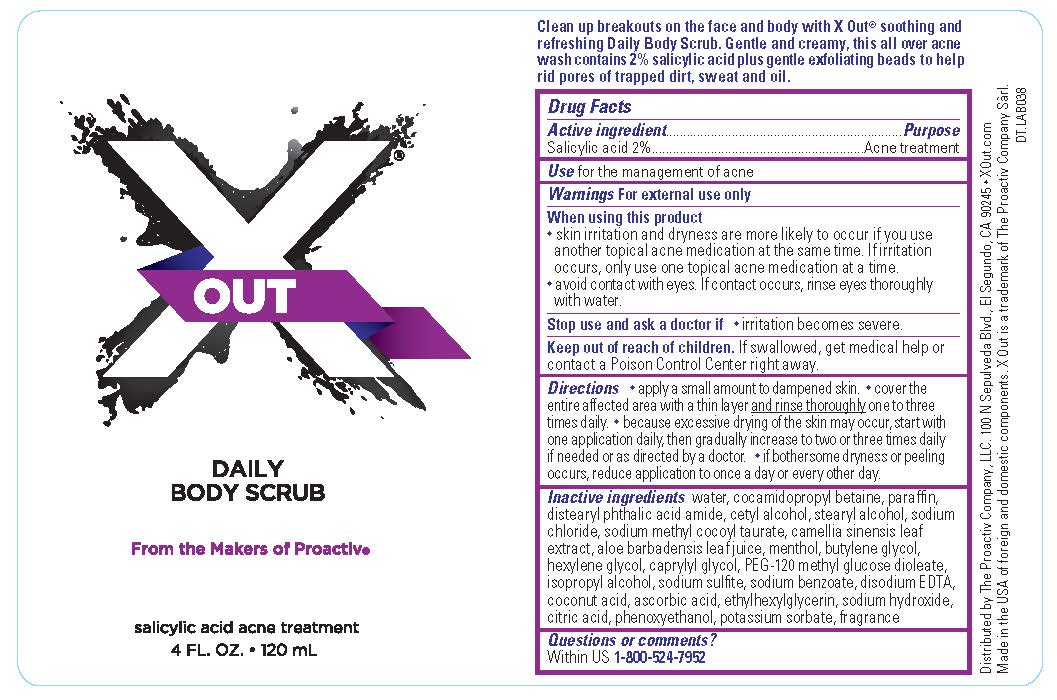
X OUT DAILY BODY SCRUB- salicylic acid cream
Alchemee, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
X Out Daily Body Scrub
Warnings
For external use only
Directions
- apply a small amount to dampened skin.
- cover the entire affected area with a thin layer and rinse thoroughly one to three times daily.
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- if bothersome dryness or peeling occurs, reduce application once a day or every other day.
Inactive Ingredients
Water, Cocamidopropyl Betaine, Paraffin, Distearyl Phthalic Acid Amide, Cetyl Alcohol, Stearyl Alcohol, Sodium Chloride, Sodium Methyl Cocoyl Taurate, Camellia Sinensis Leaf Extract, Aloe Barbadensis Leaf Juice, Menthol, Butylene Glycol, Hexylene Glycol, Caprylyl Glycol, PEG-120 Methyl Glucose Dioleate, Isopropyl Alcohol, Sodium Sulfite, Sodium Benzoate, Disodium EDTA, Coconut Acid, Ascorbic Acid, Ethylhexylglycerin, Sodium Hydroxide, Citric Acid, Phenoxyethanol, Potassium Sorbate, Fragrance
Questions or comments?
Within US 1-800-524-7952
PRINCIPAL DISPLAY PANEL - 120 mL Label
X OUT
DAILY
BODY SCRUB
From the Makers of Proactiv®
salicylic acid acne treatment
4 FL. OZ. · 120 mL
Distributed by: The Proactiv Company LLC
100 N Sepulveda Blvd., El Segundo, CA 90245
XOut.com ● Made in the USA of Foreign and Domestic Components
X Out is a trademark of The Proactiv Company Sàrl.
| X OUT DAILY BODY SCRUB
salicylic acid cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Alchemee, LLC (080216357) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| VEE PAK, LLC | 874763303 | manufacture(11410-059) | |