Label: EPI-MIST- racepinephrine hydrochloride aerosol, spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 15343-125-22 - Packager: DRNATURALHEALING INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 20, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
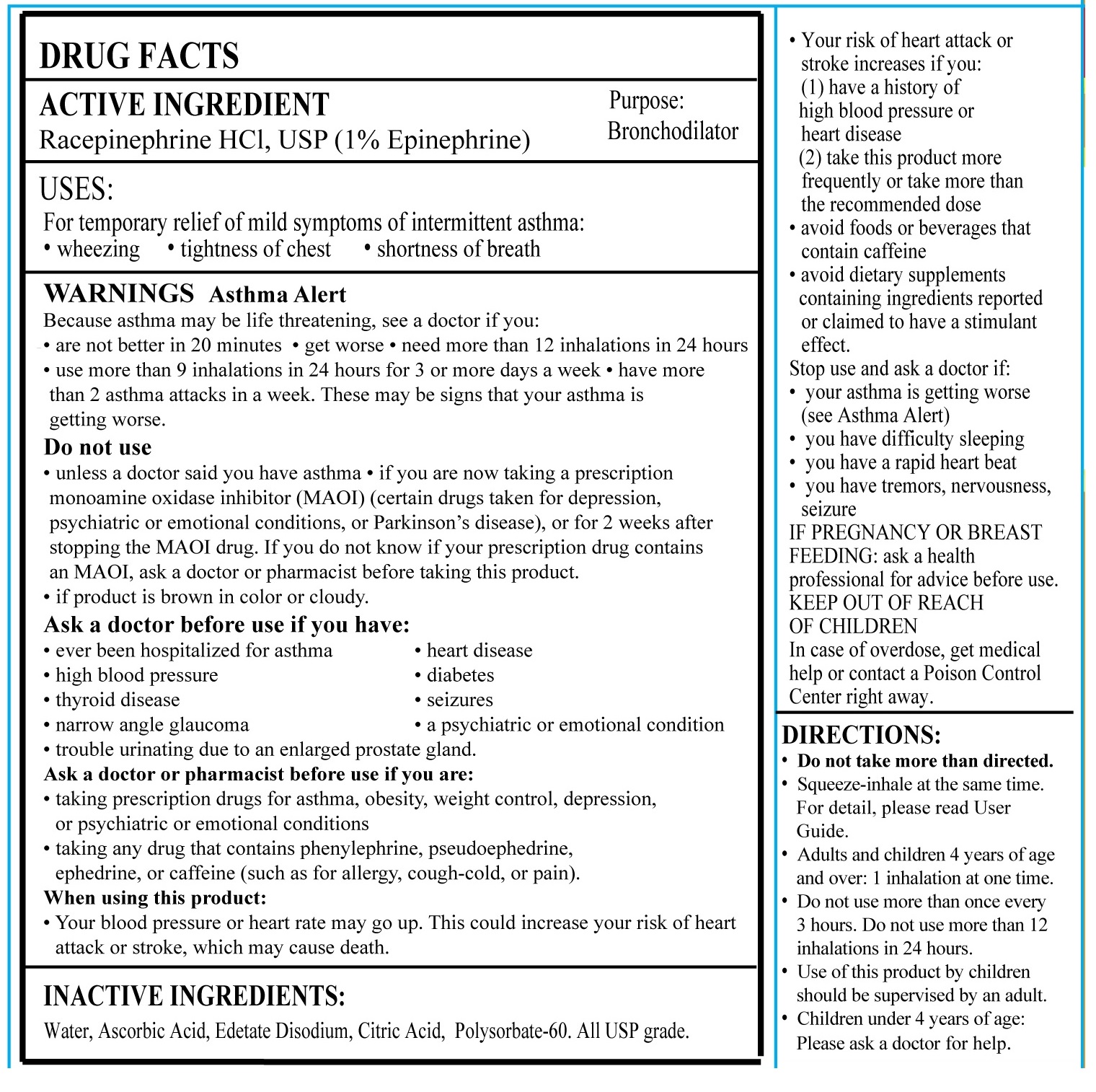
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS AND USAGE
- WARNING SECTION
-
DOSAGE AND ADMINISTRATION
DIRECTIONS
• Do not take more than directed
• Place your lips around the mouthpiece and squeeze the bulb while inhaling simultaneously to breathe in the epinephrine aerosol– see figure below for details
• Adults and children 4 years of age and over: Do not take more than one dose every 3 hours. Do not take more than 8 doses in a 24-hour period. The use of this product by children under the age of 12 should be supervised by an adult.
• For children’s usage between 4 to 12 years of age: an adult may squeeze the bulb while your child inhales.
• Please contact your doctor if your symptoms advance. - How Supplied
-
Storage and Handling
STORAGE AND HANDLING
1) Protect from light to reduce loss of the activity of the active ingredient.
2) Avoid excessive heat.
3) You can carry the whole set during daytime but avoid excessive heat and bright light.
4) When at home, it is recommended to store in refrigerator (35 to 40 oF).
5) After the liquid medicine is used out, dispose the plastic device properly. - Inactive Ingredient Section
- KEEP OUT OF REACH OF CHILDREN
- DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EPI-MIST
racepinephrine hydrochloride aerosol, sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:15343-125 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RACEPINEPHRINE HYDROCHLORIDE (UNII: 336096P2WE) (RACEPINEPHRINE - UNII:GR0L9S3J0F) RACEPINEPHRINE .594 mg in .120 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) .11808 mL in .120 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 1.08 mg in .120 mL SODIUM CITRATE (UNII: 1Q73Q2JULR) .120 mg in .120 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) .140 mg in .120 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:15343-125-22 1 in 1 KIT 03/15/2018 1 15 mL in 1 INHALER; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 05/15/2015 Labeler - DRNATURALHEALING INC (129613308) Registrant - DRNATURALHEALING INC (129613308) Establishment Name Address ID/FEI Business Operations NEW LIFE RESOURCES 169822900 manufacture(15343-125)