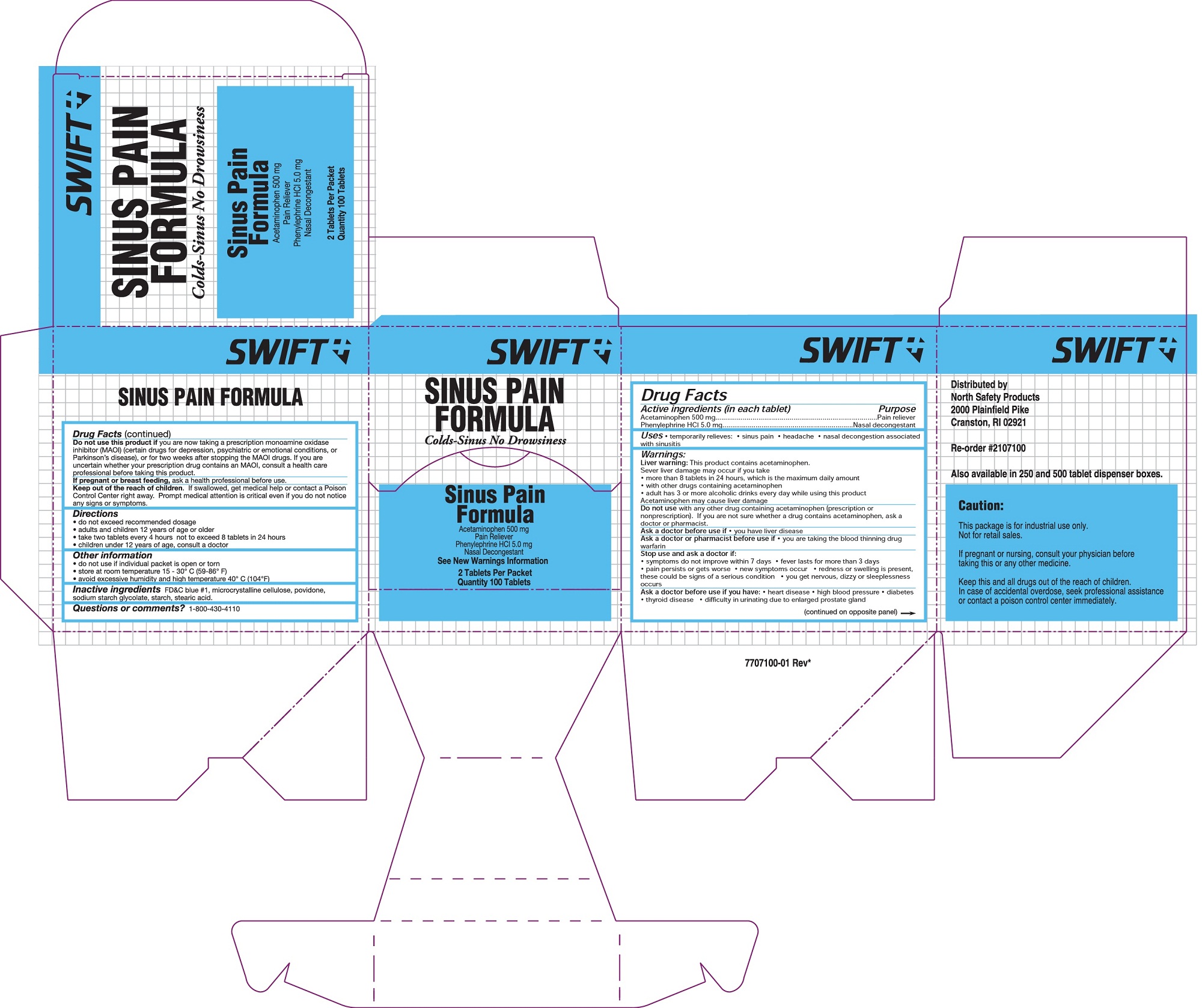
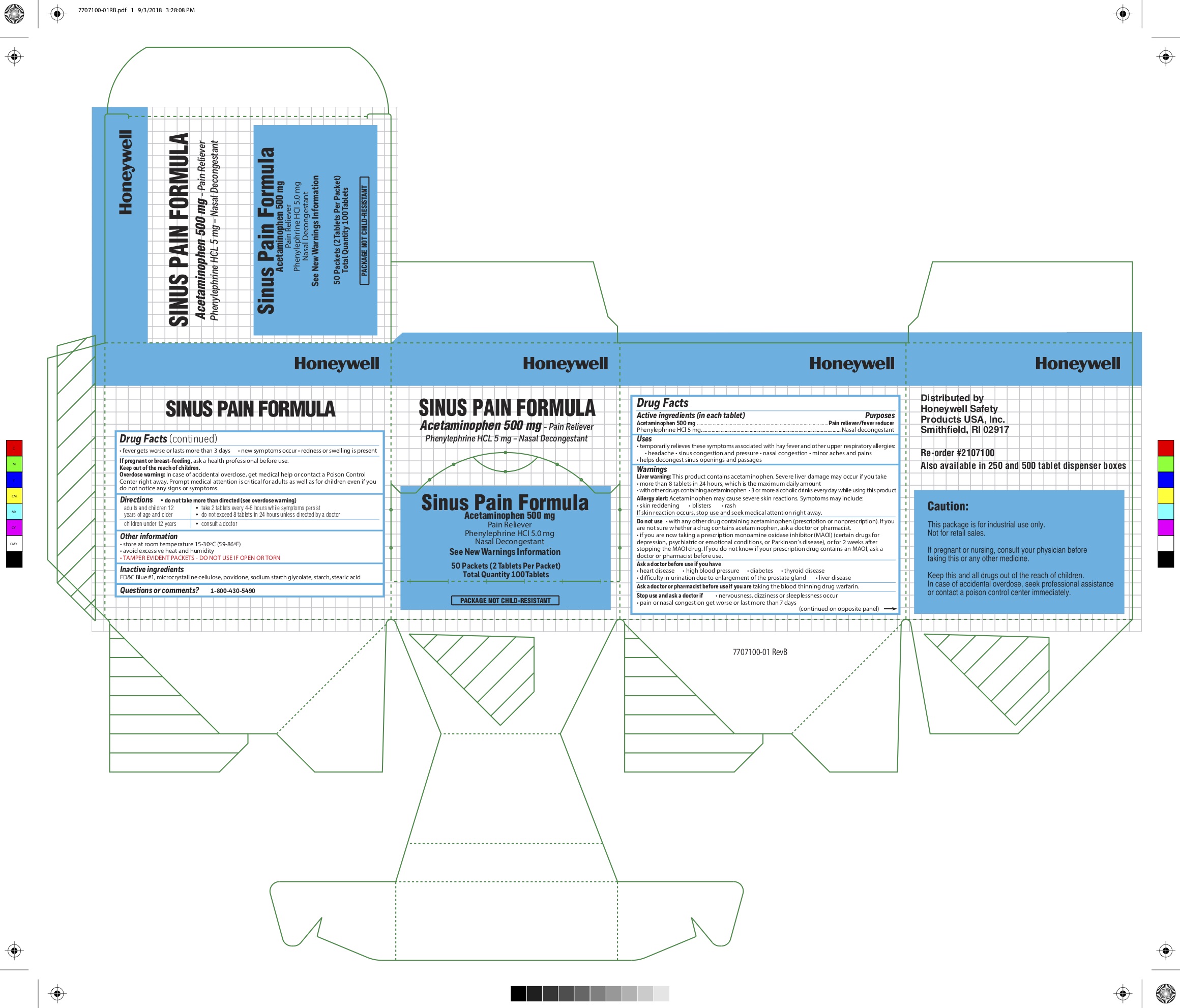
SWIFT SINUS PAIN- acetaminophen, phenylephrine hcl tablet
Honeywell Safety Products USA, Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
0498-1041 & 0498-1040: Sinus Pain
Uses
- temporarily relieves these symptoms associated with hayfever and other upper respiratory allergies
- headache
- sinus congestion and pressure
- nasal congestion
- minor aches and pains
- helps decongest sinus openings and passages
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur
- if you take more than 4,00 mg acetaminophen in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If skin reaction occurs, stop use and seek medical attention right away.
Do not use
- with any other product containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibiter (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before use.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- diabetes
- thyroid disease
- difficulty in urination due to enlargement of the prostate gland
- liver disease
Directions
- do not take morethan directed (see overdose warning)
Adults and children 12 years of age and older: take 2 tablets every 4-6 hours while symptoms persist
- do not exceed 8 tablets in 24 hours unless directed by a doctor
Children under 12 years: consult a doctor
Other Information
- store at room temperature 15-30C (59-86F)
- avoid excessive heat and humidity
- TAMPER EVIDENT PACKETS - DO NOT USE IF OPEN OR TORN
| SWIFT SINUS PAIN
acetaminophen, phenylephrine hcl tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| SINUS PAIN
acetaminophen, phenylephrine hcl tablet |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Honeywell Safety Products USA, Inc (118768815) |
Revised: 1/2024
Document Id: 0eca65ca-54d9-b264-e063-6294a90a5d40
Set id: cd167c7c-2e4a-49b9-9fec-d16c15ec77bf
Version: 13
Effective Time: 20240112
Honeywell Safety Products USA, Inc