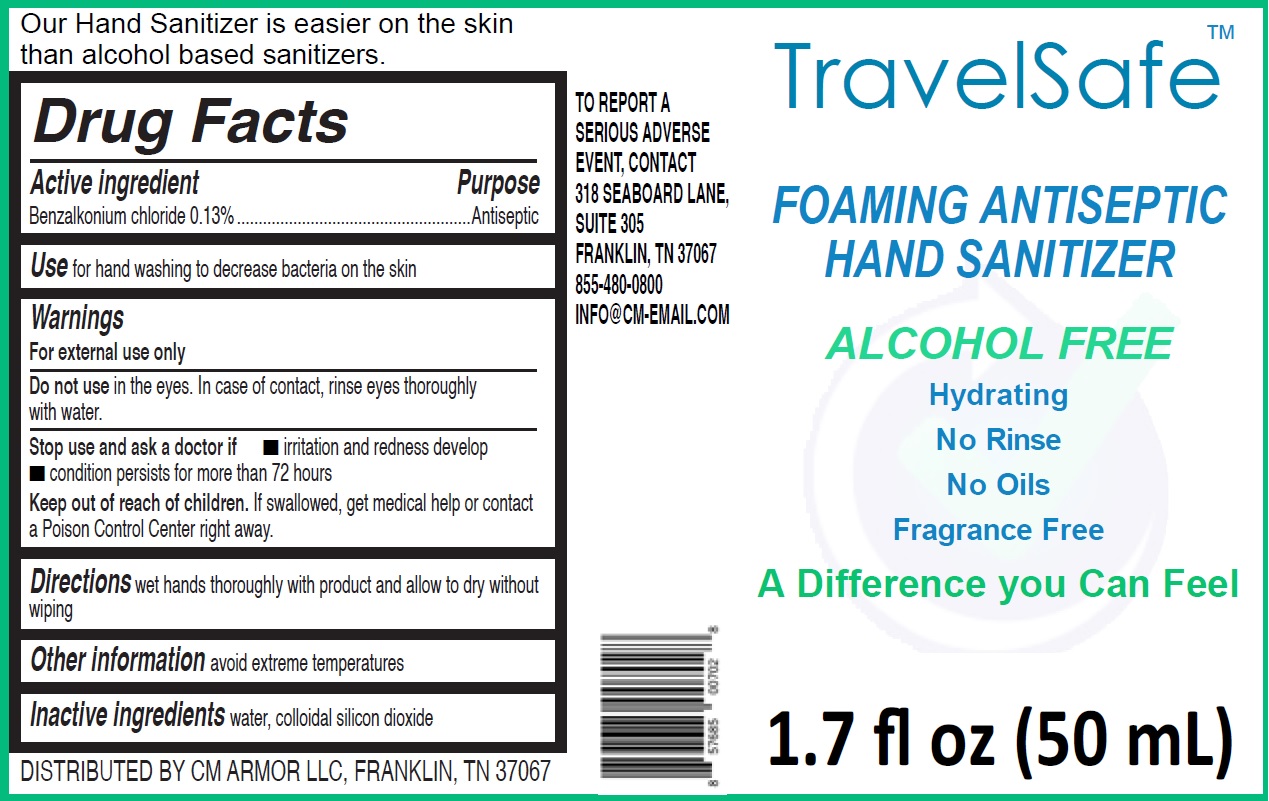
TRAVELSAFE ANTISEPTIC HAND SANITIZER FREE- benzalkonium chloride liquid
CM Armor, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
TravelSafe Foaming Antiseptic Hand Sanitizer Alcohol Free
| TRAVELSAFE ANTISEPTIC HAND SANITIZER FREE
benzalkonium chloride liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - CM Armor, LLC (080164715) |
Revised: 12/2018
Document Id: 7cb2fc38-9004-35da-e053-2a91aa0ab8e1
Set id: ccf6a419-c840-469f-b512-e8c43642ffee
Version: 3
Effective Time: 20181210
CM Armor, LLC