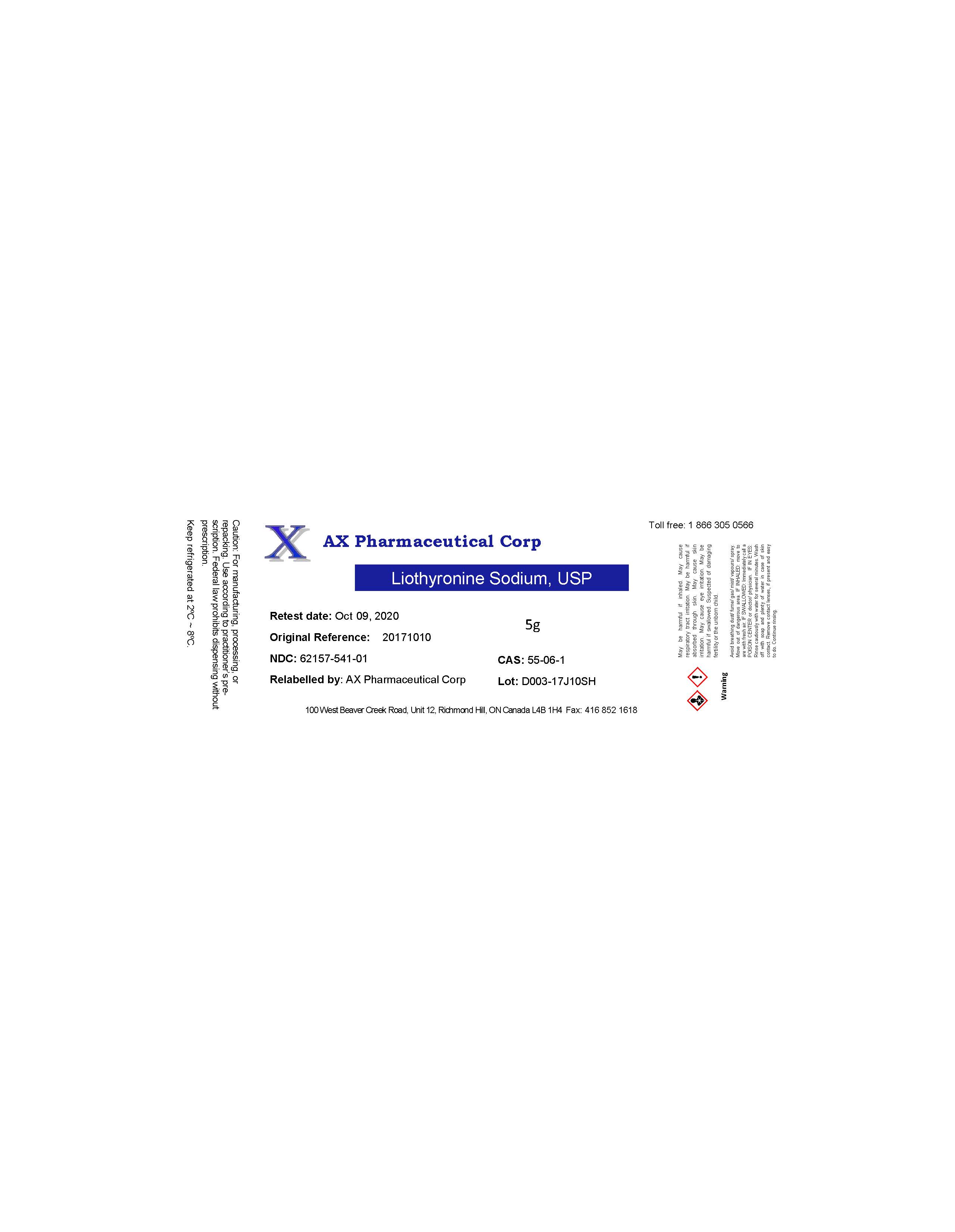
AX PHARMACEUTICAL CORP- liothyronine sodium powder
AX Pharmaceutical Corp
----------
| AX PHARMACEUTICAL CORP
liothyronine sodium powder |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - AX Pharmaceutical Corp (202924858) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AX Pharmaceutical Corp | 202924858 | repack(62157-541) , relabel(62157-541) | |
Revised: 4/2021
Document Id: a0a468ff-8da9-48d9-9c5f-65f24c628dc9
Set id: cc0f00cf-82a6-46bb-b065-c689c3aa4cd7
Version: 2
Effective Time: 20210419
AX Pharmaceutical Corp