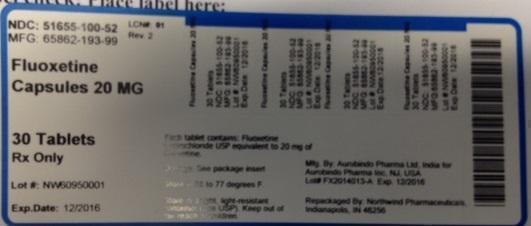
Label: FLUOXETINE HYDROCHLORIDE capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 51655-100-52 - Packager: Northwind Pharmaceuticals
- This is a repackaged label.
- Source NDC Code(s): 65862-193
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 21, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL
NDC: 51655-100-52
MFG: 65862-193-99
Fluoxetine Capsules
20 MG
30 Tablets
Rx Only
Lot# NW60950001
Exp. Date: 12/2016
Each capsule contains: Fluoxetine hydrochloride USP equivalent to 20 mg of fluoxetine
Dosage: See package insert
Store at 68 to 77 degrees F.
Store in a tight, light-resistant conainer (See USP). Keep out of the reach of children.
Mfg. by: Aurobindo Pharma Ltd, India for
Aurobindo Pharma Inc. NJ, USA
Lot# FX2014013-A exp. 12/2016
Repackaged by: Northwind Pharmaceuticals, Indianapolis, IN 46256
- WARNINGS AND PRECAUTIONS
-
INGREDIENTS AND APPEARANCE
FLUOXETINE HYDROCHLORIDE
fluoxetine hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51655-100(NDC:65862-193) Route of Administration oral Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUOXETINE HYDROCHLORIDE (UNII: I9W7N6B1KJ) (FLUOXETINE - UNII:01K63SUP8D) FLUOXETINE 20 mg Product Characteristics Color green, white Score no score Shape capsule Size 16mm Flavor Imprint Code E91 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51655-100-52 30 in 1 BOTTLE, DISPENSING Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078619 05/20/2014 Labeler - Northwind Pharmaceuticals (036986393) Registrant - Northwind Pharmaceuticals (036986393) Establishment Name Address ID/FEI Business Operations Northwind Pharmaceuticals 036986393 repack(51655-100)