URAMAXIN- urea aerosol, foam
Medimetriks Pharmaceuticals Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Uramaxin™ (20% Urea) Foam
DESCRIPTION
Uramaxin™ (20% Urea) Foam is a keratolytic emollient moisturizer. Each gram contains 20% urea, purified water, ammonium lactate, white petrolatum, octyl palmitate, caprylic/capric triglyceride, hydrogenated polyisobutene, propylene glycol, rice starch, polysorbate 60, cyclomethicone, glyceryl stearate & PEG-100 stearate, cetearyl alcohol & cetearyl glucoside, polysorbate-20, phenoxyethanol, cetyl alcohol, dimethicone, potassium sorbate, allantoin, tocopheryl acetate, xanthan gum. In propellants isobutane & propane & butane.
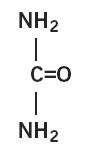
Urea is a diamide of carbonic acid with the following chemical structure:
CLINICAL PHARMACOLOGY
Urea gently lyses/dissolves the intercellular matrix of surface skin cells loosening and allowing a shedding of rough, thickened and scaly hyperkeratotic skin. Urea also moisturizes and softens skin.
INDICATIONS AND USES
For softening, smoothing and removing rough scaling hyperkeratotic skin in conditions such as xerosis, ichthyosis, skin cracks and fissures, dermatitis, eczema, psoriasis, keratoses and calluses.
CONTRAINDICATIONS
Known hypersensitivity to any of the listed ingredients. Discontinue if hypersensitivity is observed. Sun exposure to areas of skin treated with Uramaxin™ (20% Urea) Foam should be minimized or avoided.
PRECAUTIONS
This medication is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use.
PREGNANCY
Pregnancy Category C
Animal reproduction studies have not been conducted with Uramaxin™ (20% Urea) Foam. It is also not known whether Uramaxin™ (20% Urea) Foam can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Uramaxin™ (20% Urea) Foam should be given to a pregnant woman only if clearly needed.
NURSING MOTHERS
It is not known whether or not this drug is secreted in human milk. Because many drugs are secreted in human milk, caution should be exercised when Uramaxin™ (20% Urea) Foam is administered to a nursing woman.
ADVERSE REACTIONS
Transient stinging, burning, itching or irritation may occur and normally disappear on discontinuing the medication.
DOSAGE AND ADMINISTRATION
Apply Uramaxin™ (20% Urea) Foam to affected skin twice per day, or as directed by a physician. Rub in until completely absorbed.
Manufactured for:
MEDIMETRIKS PHARMACEUTICALS, INC.
363 Route 46 West
Fairfield, NJ 07004-2402 USA
www.medimetriks.com
Made in Israel
Manufactured by Perrigo
Yeruham 80500, Israel
IP005-R2
Rev. 8/10
| URAMAXIN
urea aerosol, foam |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Medimetriks Pharmaceuticals Inc (019903816) |
| Registrant - L Perrigo Company (006013346) |

