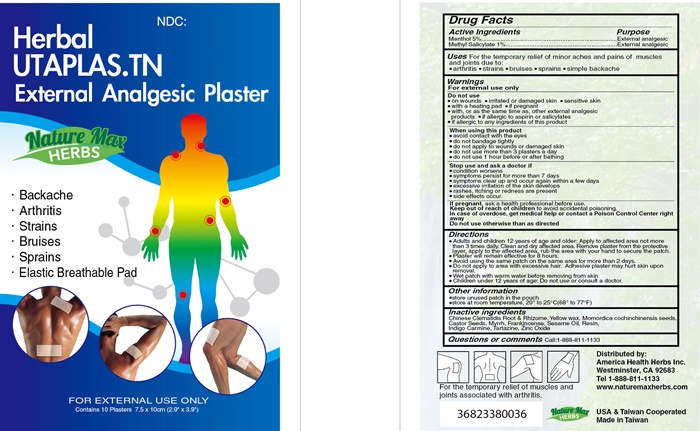
HERBAL UTAPLAS.TN- menthol, methyl salicylate plaster
Albert Max, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Herbal UTAPLAS.TN - 905
Keep out of reach of children.
Keep out of reach of children to avoid accidental poisoning.
In case of overdose, get medical help or contact a Poison Control Center right away.
Uses
For the temporary relief of minor aches and pains of muscles and joints due to:
- arthritis
- strains
- bruises
- sprains
- simple backache
Do not use
- on wounds
- irritated or damaged skin
- sensitive skin
- with a heating pad
- if pregnant
- with, or at the same time as other external analgesic products
- if allergic to aspirin or salicylates
- if allergic to any ingredients of this product
When using this product
- avoid contact with eyes
- do not bandage tightly
- do not apply to wounds or damaged skin
- do not use more than 3 plasters a day
- do not use 1 hour before or after bathing
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
- excessive irritation of the skin develops
- rashes, itching, or redness are present
- side effects occur
Directions
- Adults and children 12 years of age and older: Apply to affected area not more than 3 times daily. Clean and dry affected area. Remove plaster from the protective layer, apply to the affected area, rub the area with your hand to secure the patch.
- Plaster will remain effective for 8 hours.
- Avoid using the same patch on the same area for more than 2 days.
- Do not apply to area with excessive hair. Adhesive plaster may hurt skin upon removal.
- Wet patch with warm water beore removing from the skin.
- Children under 12 years of age: Do not use or consult a doctor.
other information
- store unused patch in the pouch
- store at room temperature, 20° to 25°C(68° to 77°F)
Inactive ingredients
Chinese Clematidis Root & Rhizome, Yellow wax, Momordica cochinchinensis seeds, Castor Seeds, Myrrh, Frankincense, Sesame Oil, Resin, Indigo Carmine, Tartazine, Zinc Oxide
Herbal UTAPLAS.TN product label
NDC:
Herbal
UTAPLAS.TN
External Analgesic Plaster
Nature Max
HERBS
- Backache
- Arthritis
- Strains
- Bruises
- Sprains
- Elastic Breathable Pad
FOR EXTERNAL USE ONLY
Contains 10 Plasters 7.5 x 10cm (2.9" x 3.9")
For the temporary relief of muscles and joints associated with arthritis.
Distributed by:
American Health herbs Inc.
Westminster, CA 92683
Tel 1-888-811-1133
www..naturemaxherbs.com
USA & Taiwan Cooperated
Made in Taiwan
| HERBAL UTAPLAS.TN
menthol, methyl salicylate plaster |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Albert Max, Inc. (149445798) |
| Registrant - Albert Max, Inc. (149445798) |