PANCREAPOWDER PLUS- pancreatic enzyme concentrate powder
Henry Schein Animal Health
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Each 2.8 g (1 teaspoon) Contains a Minimum of:
Lipase..............................71,400 USP units
Protease.........................388,000 USP units
Amylase..........................460,000 USP units
Precautions:
Discontinue use in animals with symptoms of sensitivity or allergy. May cause temporary oral mucosal irritation that is treatable by reducing dose or diluting dose with a small amount of water. Consult with your veterinarian regarding dosing.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
FOR ANIMAL USE ONLY
KEEP OUT OF THE REACH OF CHILDREN
Recommended Use:
As a digestive aid in replacement therapy where digestion of protein, carbohydrate and fat is inadequate due to exocrine pancreatic insufficiency.
Dosage:
Dose is administered before each meal and is estimated according to the severity of the condition and weight of the animal. PancreaPowder Plus is added to moistened food (canned or dry). Thorough mixing is necessary to bring the enzymes into close contact with the food particles. After mixing, let treated food stand at room temperature for 15-20 minutes prior to feeding.
Average Dose/Meal:
Dogs: 3/4-1 teaspoon (2.8 g/teaspoon), Cats: 1/4-3/4 teaspoon
Distributed by:
Henry Schein Animal Health
Dublin, OH 43017
www.henryscheinvet.com
Questions? (859)724-3461
Made in USA
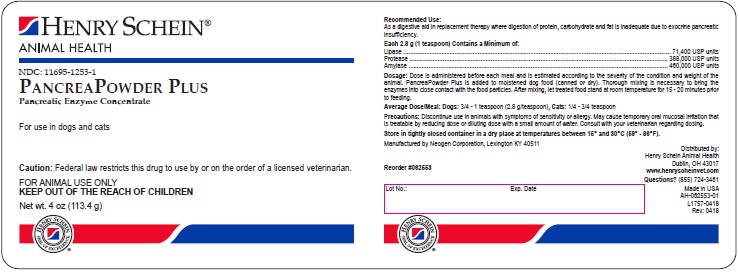
PRINCIPAL DISPLAY PANEL - 4 oz Bottle
Henry Schein®
Animal Health
NDC: 11695-1253-1
PancreaPowder Plus
Pancreatic Enzyme Concentrate
For use in dogs and cats
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
FOR ANIMAL USE ONLY
KEEP OUT OF THE REACH OF CHILDREN
Net wt. 4 oz (113.4 g)
Reorder #062553
AH-062553-01
L1757-0418
Rev: 0418
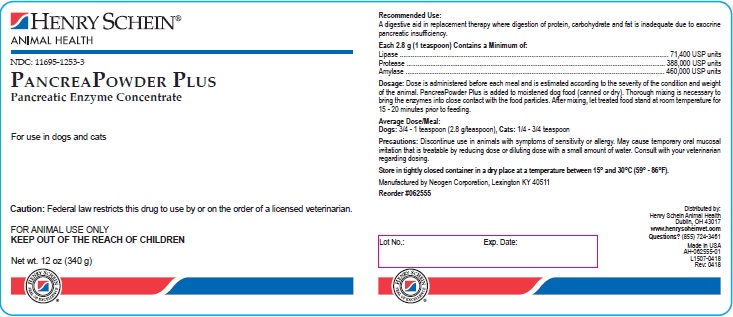
PRINCIPAL DISPLAY PANEL - 12 oz Bottle
Henry Schein®
Animal Health
NDC: 11695-1253-3
PancreaPowder Plus
Pancreatic Enzyme Concentrate
For use in dogs and cats
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
FOR ANIMAL USE ONLY
KEEP OUT OF THE REACH OF CHILDREN
Net wt. 12 oz (340 g)
Reorder #062555
AH-062555-01
L1507-0418
Rev: 0418
PRINCIPAL DISPLAY PANEL - 8 oz Bottle
Henry Schein®
Animal Health
NDC: 11695-1253-2
PancreaPowder Plus
Pancreatic Enzyme Concentrate
For use in dogs and cats
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
FOR ANIMAL USE ONLY
KEEP OUT OF THE REACH OF CHILDREN
Net wt. 8 oz (227 g)
Reorder #062554
AH-062554-01
L1756-0418
Rev: 0418

| PANCREAPOWDER PLUS
pancreatic enzyme concentrate powder |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Henry Schein Animal Health (603750329) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Neogen Corporation-Mercer | 042125879 | analysis, manufacture, label | |