CALENDULA DIAPER RASH- zinc oxide cream
Weleda AG
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
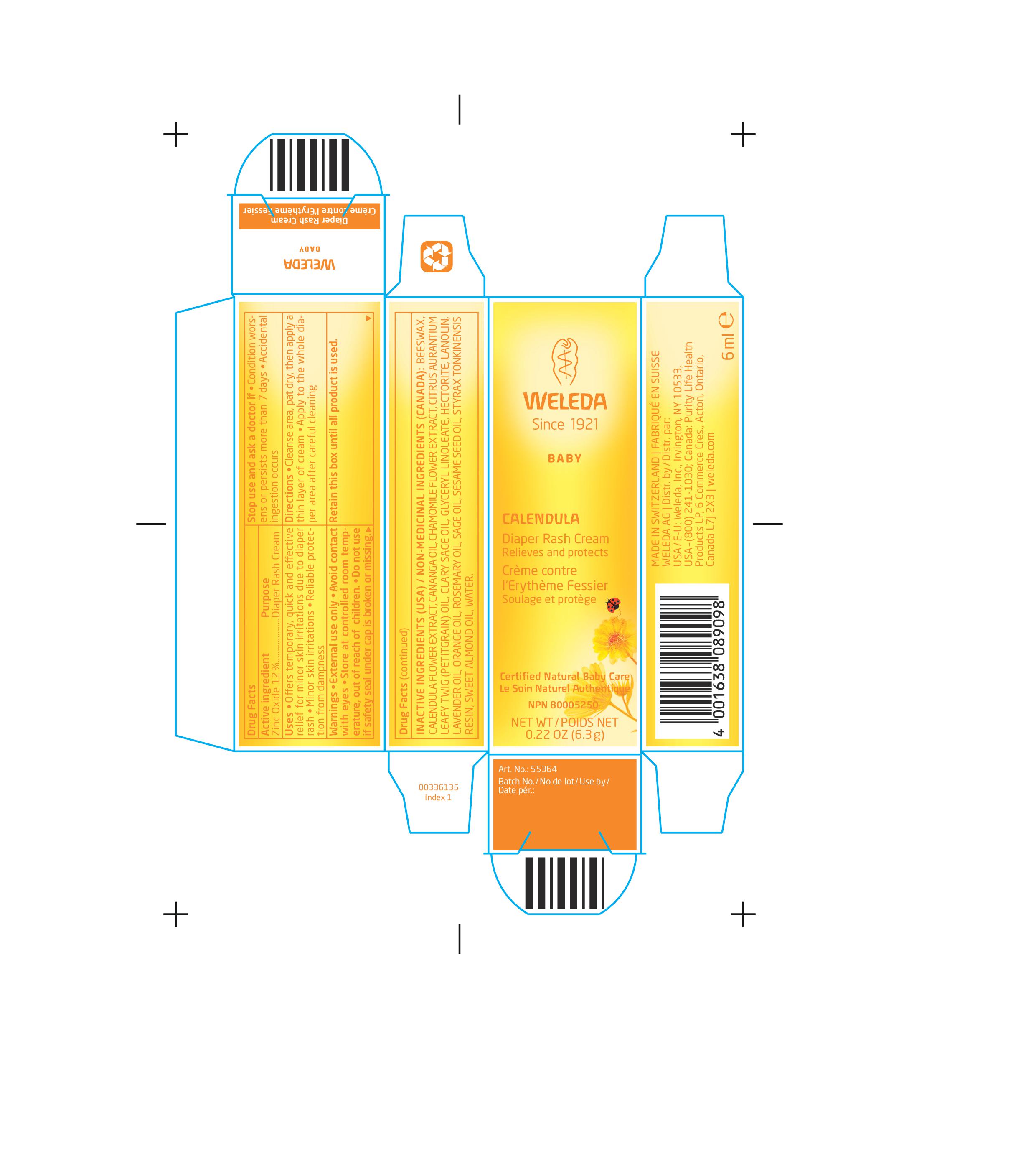
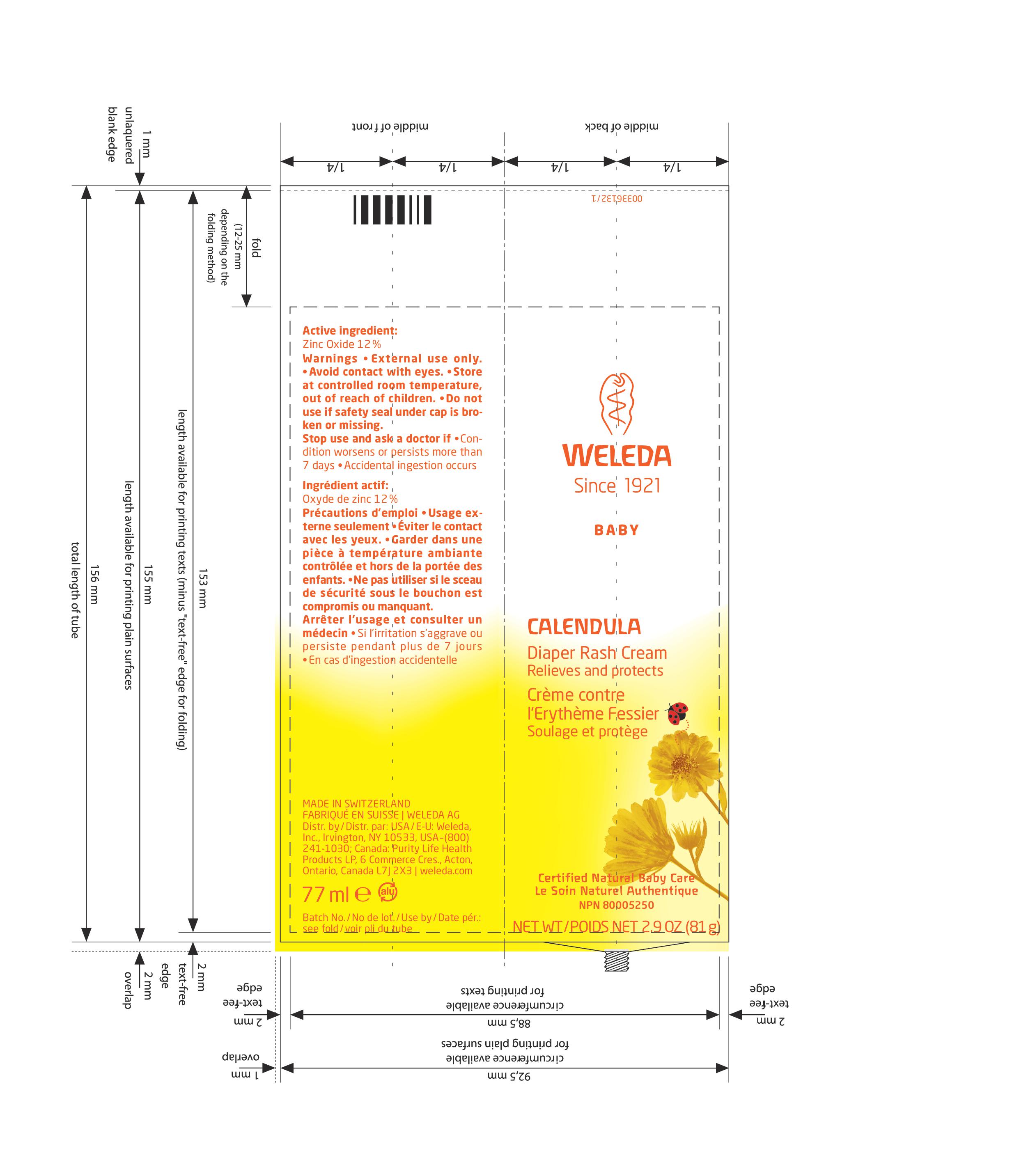
CALENDULA DIAPER RASH CREAM
Uses
Offers temporary, quick and effective relief for minor skin irritations due to diaper rash.
Minor skin irritations.
Reliable protection from dampness.
Warnings
External use only.
Avoid contact with eyes.
Store at controlled room temperature, out of reach of children.
Do not use if safety seal under cap is broken or missing.
Directions
Cleanse area, pat dry, then apply a thin layer of cream.
Apply to the whole diaper area after careful cleaning.
Inactive Ingredients (USA) / Non-Medicinal Ingredients (Canada):
Beeswax, Calendula Flower Extract, Cananga Oil, Chamomile Flower Extract, Citrus Aurantium Leafy Twig (Petitgrain) Oil, Clary Sage Oil, Glyceryl Linoleate, Hectorite, Lanolin, Lavender Oil, Orange Oil, Rosemary Oil, Sage Oil, Sesame Seed Oil, Styrax Tonkinensis Resin, Sweet Almond Oil, Water.
| CALENDULA DIAPER RASH
zinc oxide cream |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Weleda AG (480021922) |
| Registrant - Weleda, Inc. (012301297) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Weleda AG | 480021922 | manufacture(0201-4008) | |