CETAPHIL PURIFYING FACIAL BAR- triclosan soap
Galderma Laboratories, L.P.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
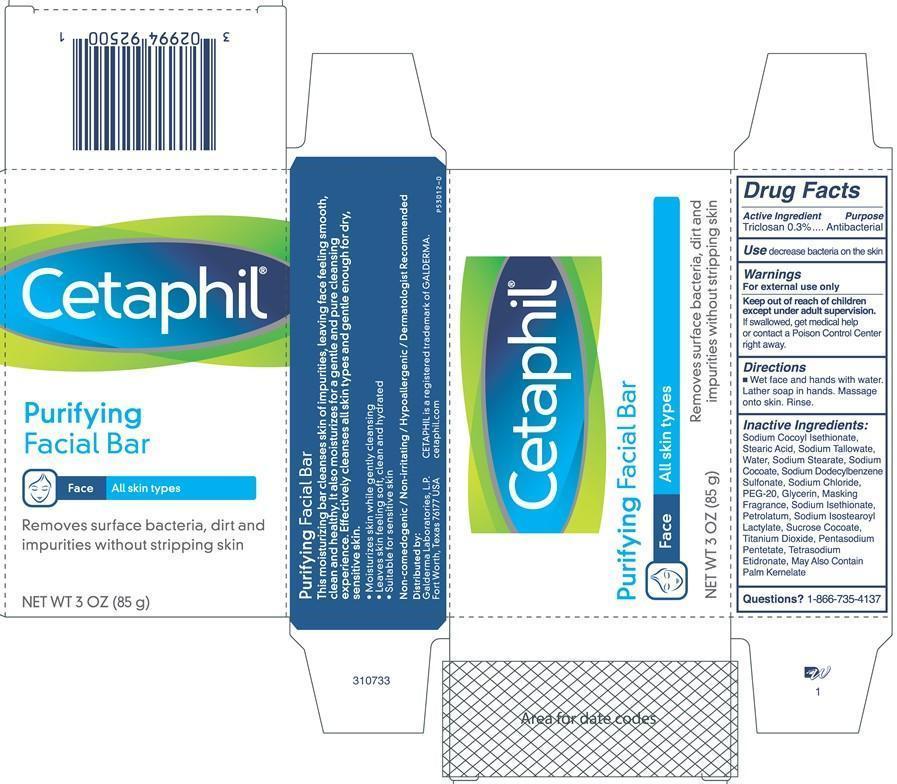
Cetaphil Purifying Facial Bar
Inactive Ingredients:
Sodium Cocoyl Isethionate, Stearic Acid, Sodium Tallowate, Water, Sodium Stearate, Sodium Cocoate, Sodium Dodecylbenzene Sulfonate, Sodium Chloride, PEG-20, Glycerin, Masking Fragrance, Sodium Isethionate, Petrolatum, Sodium Isostearoyl Lactylate, Sucrose Cocoate, Titanium Dioxide, Pentasodium Pentetate, Tetrasodium Etidronate, May Also Contain Sodium Palm Kernelate
Cetaphil
Purifying Facial Bar
Face
All skin types
Removes surface bacteria, dirt and impurities without stripping skin
NET WT 3 OZ (85 g)
Purifying Facial Bar
This moisturizing bar cleanses skin of impurities, leaving face feeling smooth, clean and healthy. It also moisturizes for a gentle and pure cleansing experience. Effectively cleanses all skin types and gentle enough for dry, sensitive skin.
Moisturizes skin while gently cleansing
Leaves skin feeling soft, clean and hydrated
Suitable for sensitive skin
Non-comedogenic / Non-irritating / Hypoallergenic / Dermatologist Recommended
Distributed by:
Galderma Laboratories, L.P.
Fort Worth, Texas 76177 USA
CETAPHIL is a registered trademark of GALDERMA.
cetaphil.com
P53012-0
Cetaphil
Purifying Facial Bar
Face
All skin types
Removes surface bacteria, dirt and impurities without stripping skin
NET WT 3 OZ (85 g)
| CETAPHIL PURIFYING FACIAL BAR
triclosan soap |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Galderma Laboratories, L.P. (047350186) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bradford Soap Works, Inc. | 001201045 | manufacture(0299-4925) | |