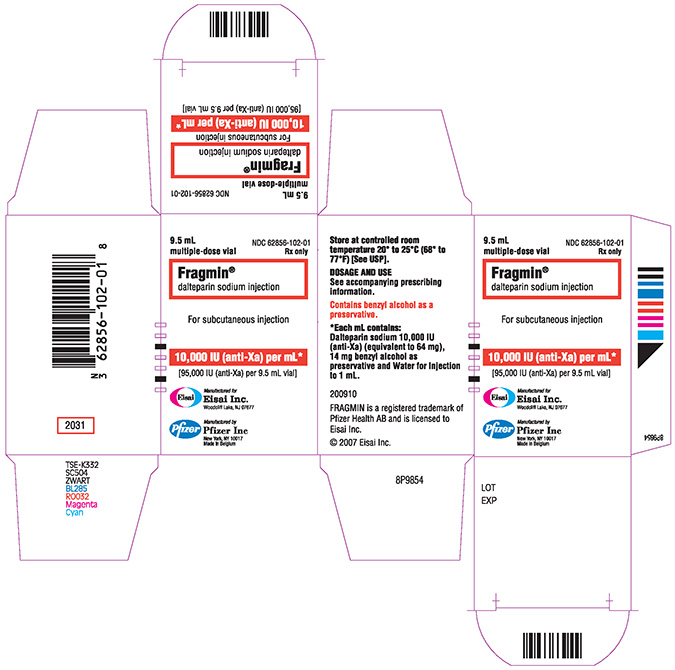
FRAGMIN- dalteparin sodium injection
Eisai Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FRAGMIN® safely and effectively. See full prescribing information for FRAGMIN.
FRAGMIN (dalteparin sodium injection) for Subcutaneous Use Only Initial U.S. Approval: 1994 WARNING: SPINAL/EPIDURAL HEMATOMASee full prescribing information for complete boxed warning.Epidural or spinal hematomas may occur in patients who are anticoagulated with low molecular weight heparins (LMWH) or heparinoids and are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary. Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis [see Warnings and Precautions (5.1) and Drug Interactions (7)]. RECENT MAJOR CHANGESBoxed Warning 1/2015 Risk of Hemorrhage including Spinal / Epidural Hematoma (5.1) 1/2015 INDICATIONS AND USAGEFRAGMIN is a low molecular weight heparin [LMWH] indicated for
Limitations of Use DOSAGE AND ADMINISTRATION
Do not use as intramuscular injection. FRAGMIN should not be mixed with other injections or infusions. (2) DOSAGE FORMS AND STRENGTHSCONTRAINDICATIONS
WARNINGS AND PRECAUTIONSADVERSE REACTIONSMost common adverse reaction is hematoma at the injection site. (6)
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION. Revised: 1/2015 |
FULL PRESCRIBING INFORMATION
WARNING: SPINAL/EPIDURAL HEMATOMAS
Epidural or spinal hematomas may occur in patients who are anticoagulated with low molecular weight heparins (LMWH) or heparinoids and are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
- Use of indwelling epidural catheters
- Concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants.
- A history of traumatic or repeated epidural or spinal punctures
- A history of spinal deformity or spinal surgery
- Optimal timing between the administration of FRAGMIN and neuraxial procedures is not known
Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary.
Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis [see Warnings and Precautions (5.1) and Drug Interactions (7)].
1 INDICATIONS AND USAGE
1.1 Prophylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction
FRAGMIN® Injection is indicated for the prophylaxis of ischemic complications in unstable angina and non-Q-wave myocardial infarction, when concurrently administered with aspirin therapy [see Clinical Studies (14.1)].
1.2 Prophylaxis of Deep Vein Thrombosis
FRAGMIN is also indicated for the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE):
- In patients undergoing hip replacement surgery [see Clinical Studies (14.2)];
- In patients undergoing abdominal surgery who are at risk for thromboembolic complications [see Clinical Studies (14.3)];
- In medical patients who are at risk for thromboembolic complications due to severely restricted mobility during acute illness [see Clinical Studies (14.4)].
1.3 Extended Treatment of Symptomatic Venous Thromboembolism in Patients with Cancer
FRAGMIN is also indicated for the extended treatment of symptomatic venous thromboembolism (VTE) (proximal DVT and/or PE), to reduce the recurrence of VTE in patients with cancer. In these patients, the FRAGMIN therapy begins with the initial VTE treatment and continues for six months [see Clinical Studies (14.5)].
Limitations of Use
FRAGMIN is not indicated for the acute treatment of VTE.
2 DOSAGE AND ADMINISTRATION
FRAGMIN is administered by subcutaneous injection. It must not be administered by intramuscular injection.
FRAGMIN Injection should not be mixed with other injections or infusions unless specific compatibility data are available that support such mixing.
Routine coagulation tests such as Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT) are relatively insensitive measures of FRAGMIN activity and, therefore, unsuitable for monitoring the anticoagulant effect of FRAGMIN. [see Warnings and Precautions (5)].
2.1 Adult Dosage
Prophylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction: In patients with unstable angina or non-Q-wave myocardial infarction, the recommended dose of FRAGMIN Injection is 120 IU/kg of body weight, but not more than 10,000 IU, subcutaneously every 12 hours with concurrent oral aspirin (75 to 165 mg once daily) therapy. Treatment should be continued until the patient is clinically stabilized. The usual duration of administration is 5 to 8 days. Concurrent aspirin therapy is recommended except when contraindicated.
Table 1 lists the volume of FRAGMIN, based on the 9.5 mL multiple-dose vial (10,000 IU/mL), to be administered for a range of patient weights.
| Table 1 | ||||||
| Volume of FRAGMIN to be Administered by Patient Weight, Based on 9.5 mL Vial (10,000 IU/mL) |
||||||
| Patient weight (lb) | < 110 | 110 to 131 | 132 to 153 | 154 to 175 | 176 to 197 | ≥ 198 |
| Patient weight (kg) | < 50 | 50 to 59 | 60 to 69 | 70 to 79 | 80 to 89 | ≥ 90 |
| Volume of FRAGMIN (mL) | 0.55 | 0.65 | 0.75 | 0.90 | 1.0 | 1.0 |
Prophylaxis of Venous Thromboembolism Following Hip Replacement Surgery: Table 2 presents the dosing options for patients undergoing hip replacement surgery. The usual duration of administration is 5 to 10 days after surgery; up to 14 days of treatment with FRAGMIN have been well tolerated in clinical trials.
| Table 2 | ||||
| Dosing Options for Patients Undergoing Hip Replacement Surgery | ||||
| Timing of First Dose of FRAGMIN | Dose of FRAGMIN to be Given Subcutaneously | |||
| 10 to 14 Hours Before Surgery | Within 2 Hours Before Surgery | 4 to 8 Hours After Surgery1 | Postoperative Period2 |
|
| Postoperative Start | --- | --- | 2500 IU3 | 5000 IU once daily |
| Preoperative Start - Day of Surgery | --- | 2500 IU | 2500 IU3 | 5000 IU once daily |
| Preoperative Start - Evening Before Surgery4 | 5000 IU | --- | 5000 IU | 5000 IU once daily |
1 Or later, if hemostasis has not been achieved.
2 Up to 14 days of treatment was well tolerated in controlled clinical trials, where the usual duration of treatment was 5 to 10 days postoperatively.
3 Allow a minimum of 6 hours between this dose and the dose to be given on Postoperative Day 1. Adjust the timing of the dose on Postoperative Day 1 accordingly.
4 Allow approximately 24 hours between doses.
Abdominal Surgery: In patients undergoing abdominal surgery with a risk of thromboembolic complications, the recommended dose of FRAGMIN is 2500 IU administered by subcutaneous injection once daily, starting 1 to 2 hours prior to surgery and repeated once daily postoperatively. The usual duration of administration is 5 to 10 days.
In patients undergoing abdominal surgery associated with a high risk of thromboembolic complications, such as malignant disorder, the recommended dose of FRAGMIN is 5000 IU subcutaneously the evening before surgery, then once daily postoperatively. The usual duration of administration is 5 to 10 days. Alternatively, in patients with malignancy, 2500 IU of FRAGMIN can be administered subcutaneously 1 to 2 hours before surgery followed by 2500 IU subcutaneously 12 hours later, and then 5000 IU once daily postoperatively. The usual duration of administration is 5 to 10 days.
Medical Patients During Acute Illness: In medical patients with severely restricted mobility during acute illness, the recommended dose of FRAGMIN is 5000 IU administered by subcutaneous injection once daily. In clinical trials, the usual duration of administration was 12 to 14 days.
Extended Treatment of Symptomatic Venous Thromboembolism in Patients with Cancer: In patients with cancer and symptomatic venous thromboembolism, the recommended dosing of FRAGMIN is as follows: for the first 30 days of treatment administer FRAGMIN 200 IU/kg total body weight subcutaneously once daily. The total daily dose should not exceed 18,000 IU. Table 3 lists the dose of FRAGMIN to be administered once daily during the first month for a range of patient weights.
Month 1
| Table 3 | ||
| Dose of FRAGMIN to be Administered Subcutaneously by Patient Weight during the First Month |
||
| Body Weight (lbs) | Body Weight (kg) | FRAGMIN Dose (IU) (prefilled syringe) once daily |
| ≤ 124 | ≤ 56 | 10,000 |
| 125 to 150 | 57 to 68 | 12,500 |
| 151 to 181 | 69 to 82 | 15,000 |
| 182 to 216 | 83 to 98 | 18,000 |
| ≥ 217 | ≥ 99 | 18,000 |
Months 2 to 6
Administer FRAGMIN at a dose of approximately 150 IU/kg, subcutaneously once daily during Months 2 through 6. The total daily dose should not exceed 18,000 IU. Table 4 lists the dose of FRAGMIN to be administered once daily for a range of patient weights during months 2-6.
| Table 4 | ||
| Dose of FRAGMIN to be Administered Subcutaneously by Patient Weight during Months 2-6 |
||
| Body Weight (lbs) | Body Weight (kg) | FRAGMIN Dose (IU) (prefilled syringe) once daily |
| ≤ 124 | ≤ 56 | 7,500 |
| 125 to 150 | 57 to 68 | 10,000 |
| 151 to 181 | 69 to 82 | 12,500 |
| 182 to 216 | 83 to 98 | 15,000 |
| ≥ 217 | ≥ 99 | 18,000 |
Safety and efficacy beyond six months have not been evaluated in patients with cancer and acute symptomatic VTE [see Warnings and Precaution (5) and Adverse Reactions (6.1)].
2.2 Dose Reductions for Thrombocytopenia in Patients with Cancer and Acute Symptomatic VTE
In patients receiving FRAGMIN who experience platelet counts between 50,000 and 100,000/mm3, reduce the daily dose of FRAGMIN by 2,500 IU until the platelet count recovers to ≥ 100,000/mm3. In patients receiving FRAGMIN who experience platelet counts < 50,000/mm3, discontinue FRAGMIN until the platelet count recovers above 50,000/mm3.
2.3 Dose Reductions for Renal Insufficiency in Extended Treatment of Acute Symptomatic Venous Thromboembolism in Patients with Cancer
In patients with severely impaired renal function (CrCl < 30 mL/min), monitor anti-Xa levels to determine the appropriate FRAGMIN dose. Target anti-Xa range is 0.5-1.5 IU/mL. When monitoring anti-Xa in these patients, perform sampling 4-6 hrs after FRAGMIN dosing and only after the patient has received 3-4 doses.
2.4 Administration
Subcutaneous injection technique: Patients should be sitting or lying down and FRAGMIN administered by deep subcutaneous injection. FRAGMIN may be injected in a U-shape area around the navel, the upper outer side of the thigh or the upper outer quadrangle of the buttock. The injection site should be varied daily. When the area around the navel or the thigh is used, using the thumb and forefinger, you must lift up a fold of skin while giving the injection. The entire length of the needle should be inserted at a 45 to 90 degree angle.
Inspect FRAGMIN prefilled syringes and vials visually for particulate matter and discoloration prior to administration
After first penetration of the rubber stopper, store the multiple-dose vials at room temperature for up to 2 weeks. Discard any unused solution after 2 weeks.
Instructions for using the prefilled single-dose syringes preassembled with needle guard devices
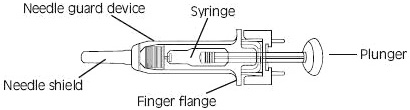
Fixed dose syringes: To ensure delivery of the full dose, do not expel the air bubble from the prefilled syringe before injection. Hold the syringe assembly by the open sides of the device. Remove the needle shield. Insert the needle into the injection area as instructed above. Depress the plunger of the syringe while holding the finger flange until the entire dose has been given. The needle guard will not be activated unless the entire dose has been given. Remove needle from the patient. Let go of the plunger and allow syringe to move up inside the device until the entire needle is guarded. Discard the syringe assembly in approved containers.
Graduated syringes: Hold the syringe assembly by the open sides of the device. Remove the needle shield. With the needle pointing up, prepare the syringe by expelling the air bubble and then continuing to push the plunger to the desired dose or volume, discarding the extra solution in an appropriate manner. Insert the needle into the injection area as instructed above. Depress the plunger of the syringe while holding the finger flange until the entire dose remaining in the syringe has been given. The needle guard will not be activated unless the entire dose has been given. Remove needle from the patient. Let go of the plunger and allow syringe to move up inside the device until the entire needle is guarded. Discard the syringe assembly in approved containers.
3 DOSAGE FORMS AND STRENGTHS
2,500 IU / 0.2 mL single-dose prefilled syringe
5,000 IU / 0.2 mL single-dose prefilled syringe
7,500 IU / 0.3 mL single-dose prefilled syringe
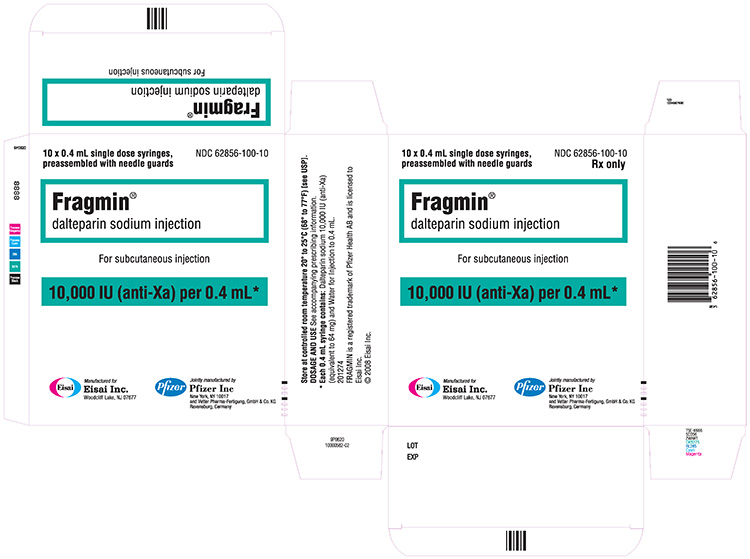
10,000 IU / 0.4 mL single-dose prefilled syringe
10,000 IU / 1 mL single-dose graduated syringe
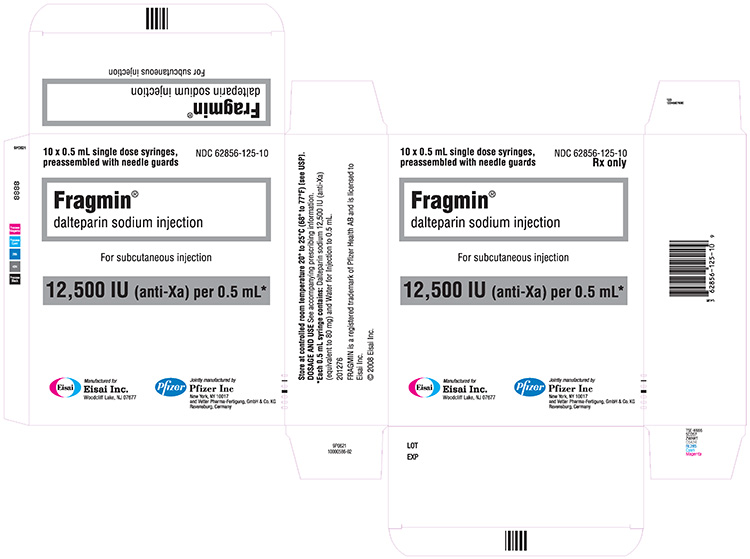
12,500 IU / 0.5 mL single-dose prefilled syringe
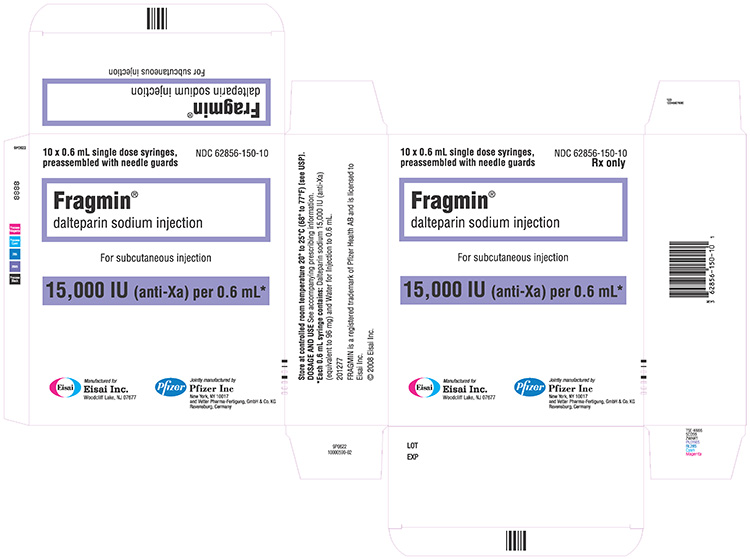
15,000 IU / 0.6 mL single-dose prefilled syringe
18,000 IU / 0.72 mL single-dose prefilled syringe
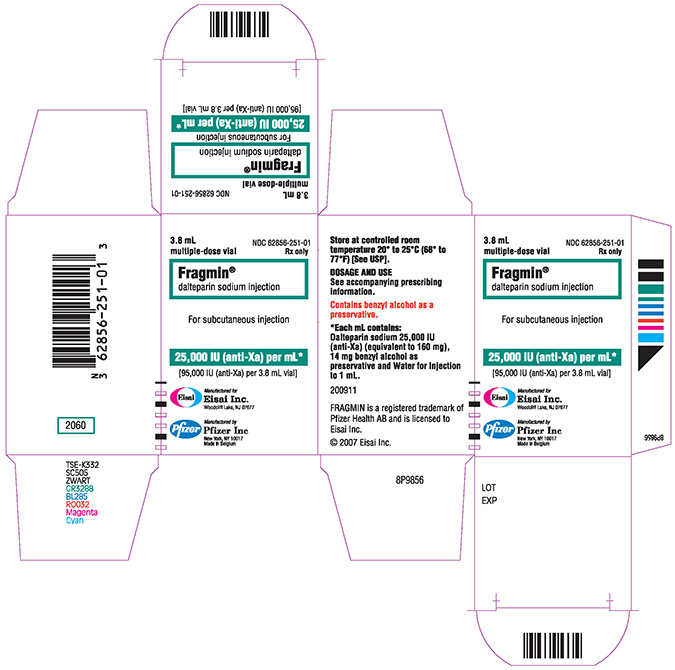
95,000 IU / 3.8 mL multiple-dose vial
95,000 IU / 9.5 mL multiple-dose vial
4 CONTRAINDICATIONS
- Active major bleeding
- History of heparin induced thrombocytopenia or heparin induced thrombocytopenia with thrombosis.
- Hypersensitivity to dalteparin sodium (e.g., pruritis, rash, anaphylactic reactions) [see Adverse Reactions (6.2)]
- In patients undergoing Epidural/Neuraxial anesthesia, do not administer FRAGMIN [see Boxed Warning, Warnings and Precautions (5.1)];
- As a treatment for unstable angina and non-Q-wave MI.
- For prolonged VTE prophylaxis.
- Hypersensitivity to heparin or pork products
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Hemorrhage including Spinal / Epidural Hematoma
Spinal or epidural hemorrhage and subsequent hematomas can occur with the associated use of low molecular weight heparins or heparinoids and neuraxial (spinal/epidural) anesthesia or spinal puncture. The risk of these events is higher with the use of post-operative indwelling epidural catheters, with the concomitant use of additional drugs affecting hemostasis such as NSAIDs, with traumatic or repeated epidural or spinal puncture, or in patients with a history of spinal surgery or spinal deformity [see Boxed Warning and Adverse Reactions (6.2) and Drug Interactions (7)].
To reduce the potential risk of bleeding associated with the concurrent use of dalteparin sodium and epidural or spinal anesthesia/analgesia or spinal puncture, consider the pharmacokinetic profile of dalteparin [see Clinical Pharmacology (12.3)].
Placement or removal of an epidural catheter or lumbar puncture is best performed when the anticoagulant effect of dalteparin is low; however, the exact timing to reach a sufficiently low anticoagulant effect in each patient is not known. No additional hemostasis-altering medications should be administered due to the additive effects.
Patients on preoperative FRAGMIN thromboprophylaxis can be assumed to have altered coagulation. The first postoperative FRAGMIN thrombophylaxis dose (2500 IU) should be administered 6 to 8 hrs postoperatively. The second postoperative dose (2500 or 5000 IU) should occur no sooner than 24 hrs after the first dose. Placement or removal of a catheter should be delayed for at least 12 hours after administration of 2500 IU once daily of FRAGMIN, at least 15 hours after the administration of 5000 IU once daily of FRAGMIN, and at least 24 hours after the administration of higher doses (200 IU/kg once daily, 120 IU/kg twice daily) of FRAGMIN. Anti-Xa levels are still detectable at these time points, and these delays are not a guarantee that neuraxial hematoma will be avoided.
Although a specific recommendation for timing of a subsequent FRAGMIN dose after catheter removal cannot be made, consider delaying this next dose for at least four hours, based on a benefit-risk assessment considering both the risk for thrombosis and the risk for bleeding in the context of the procedure and patient risk factors. For patients with creatinine clearance <30mL/minute, additional considerations are necessary because elimination of FRAGMIN may be more prolonged; consider doubling the timing of removal of a catheter, at least 24 hours for the lower prescribed dose of FRAGMIN (2500 IU or 5000 IU once daily) and at least 48 hours for the higher dose (200 IU/kg once daily, 120 IU/kg twice daily) [see Clinical Pharmacology (12.3)].
Should the physician decide to administer anticoagulation in the context of epidural or spinal anesthesia/analgesia or lumbar puncture, frequent monitoring must be exercised to detect any signs and symptoms of neurological impairment such as midline back pain, sensory and motor deficits (numbness or weakness in lower limbs), bowel and/or bladder dysfunction. Instruct patients to report immediately if they experience any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, initiate urgent diagnosis and treatment including consideration for spinal cord decompression even though such treatment may not prevent or reverse neurological sequelae.
Use FRAGMIN with extreme caution in patients who have an increased risk of hemorrhage, such as those with severe uncontrolled hypertension, bacterial endocarditis, congenital or acquired bleeding disorders, active ulceration and angiodysplastic gastrointestinal disease, hemorrhagic stroke, or shortly after brain, spinal or ophthalmological surgery. Fragmin may enhance the risk of bleeding in patients with thrombocytopenia or platelet defects; severe liver or kidney insufficiency, hypertensive or diabetic retinopathy, and recent gastrointestinal bleeding. Bleeding can occur at any site during therapy with FRAGMIN.
5.2 Thrombocytopenia
Heparin-induced thrombocytopenia can occur with the administration of FRAGMIN. The incidence of this complication is unknown at present. In clinical practice, cases of thrombocytopenia with thrombosis, amputation and death have been observed. [see Contraindications (4)] Closely monitor thrombocytopenia of any degree.
In FRAGMIN clinical trials supporting non-cancer indications, platelet counts of < 50,000/mm3 occurred in < 1% of patients.
In the clinical trial of patients with cancer and acute symptomatic venous thromboembolism treated for up to 6 months in the FRAGMIN treatment arm, platelet counts of < 100,000/mm3 occurred in 13.6% of patients, including 6.5% who also had platelet counts less than 50,000/mm3. In the same clinical trial, thrombocytopenia was reported as an adverse event in 10.9% of patients in the FRAGMIN arm and 8.1% of patients in the OAC arm. FRAGMIN dose was decreased or interrupted in patients whose platelet counts fell below 100,000/mm3.
5.3 Benzyl Alcohol
Each multiple-dose vial of FRAGMIN contains benzyl alcohol as a preservative. Benzyl alcohol has been reported to be associated with a fatal "Gasping Syndrome" in premature infants. Because benzyl alcohol may cross the placenta, use caution when administering FRAGMIN preserved with benzyl alcohol to pregnant women. If anticoagulation with FRAGMIN is needed during pregnancy, use preservative-free formulations, where possible. [see Use in Specific Populations (8.1)].
5.4 Laboratory Tests
Periodic routine complete blood counts, including platelet count, blood chemistry, and stool occult blood tests are recommended during the course of treatment with FRAGMIN. When administered at recommended prophylaxis doses, routine coagulation tests such as Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT) are relatively insensitive measures of FRAGMIN activity and, therefore, unsuitable for monitoring the anticoagulant effect of FRAGMIN. Anti-Factor Xa may be used to monitor the anticoagulant effect of FRAGMIN, such as in patients with severe renal impairment or if abnormal coagulation parameters or bleeding occurs during FRAGMIN therapy.
6 ADVERSE REACTIONS
The following serious adverse reactions are described in more detail in other sections of the prescribing information.
- Risk of Hemorrhage including Spinal/Epidural Hematoma [see Warnings and Precautions (5.1)]
- Thrombocytopenia [see Warnings and Precautions (5.2)]
- Benzyl Alcohol preservative Risk to Premature Infants [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not accurately reflect the rates observed in practice.
Hemorrhage
The incidence of hemorrhagic complications during treatment with FRAGMIN Injection has been low. The most commonly reported side effect is hematoma at the injection site. The risk for bleeding varies with the indication and may increase with higher doses.
Unstable Angina and Non-Q-Wave Myocardial Infarction
Table 5 summarizes major bleeding reactions that occurred with FRAGMIN, heparin, and placebo in clinical trials of unstable angina and non-Q-wave myocardial infarction.
| Table 5 | |||
| Major Bleeding Reactions in Unstable Angina and Non-Q-Wave Myocardial Infarction |
|||
| Indication | Dosing Regimen | ||
| Unstable Angina and Non-Q-Wave MI | FRAGMIN
120 IU/kg/12 hr subcutaneous1 n (%) | Heparin2
intravenous and subcutaneous2 n (%) | Placebo
every 12 hr subcutaneous n (%) |
| Major Bleeding Reactions3,4 | 15/1497 (1.0) | 7/731 (1.0) | 4/760 (0.5) |
1 Treatment was administered for 5 to 8 days.
2 Heparin intravenous infusion for at least 48 hours, APTT 1.5 to 2 times control, then 12,500 U subcutaneously every 12 hours for 5 to 8 days.
3 Aspirin (75 to 165 mg per day) and beta blocker therapies were administered concurrently
4 Bleeding reactions were considered major if: 1) accompanied by a decrease in hemoglobin of ≥2 g/dL in connection with clinical symptoms; 2) a transfusion was required; 3) bleeding led to interruption of treatment or death; or 4) intracranial bleeding.
Hip Replacement Surgery
Table 6 summarizes: 1) all major bleeding reactions and, 2) other bleeding reactions possibly or probably related to treatment with FRAGMIN (preoperative dosing regimen), warfarin sodium, or heparin in two hip replacement surgery clinical trials.
| Table 6 | ||||
| Bleeding Reactions Following Hip Replacement Surgery | ||||
| Indication | FRAGMIN vs Warfarin Sodium | FRAGMIN vs Heparin |
||
| Dosing Regimen | Dosing Regimen | |||
| Hip Replacement Surgery | FRAGMIN2
5000 IU once daily subcutaneous n (%) | Warfarin
Sodium1 oral n (%) | FRAGMIN4
5000 IU once daily subcutaneous n (%) | Heparin
5000 U three times a day subcutaneous n (%) |
| Major Bleeding Reactions3 | 7/274 (2.6) | 1/279 (0.4) | 0 | 3/69 (4.3) |
| Other Bleeding Reactions5 Hematuria | 8/274 (2.9) | 5/279 (1.8) | 0 | 0 |
| Wound Hematoma | 6/274 (2.2) | 0 | 0 | 0 |
| Injection Site Hematoma | 3/274 (1.1) | NA | 2/69 (2.9) | 7/69 (10.1) |
1 Warfarin sodium dosage was adjusted to maintain a prothrombin time index of 1.4 to 1.5, corresponding to an International Normalized Ratio (INR) of approximately 2.5.
2 Includes three treated patients who did not undergo a surgical procedure.
3 A bleeding event was considered major if: 1) hemorrhage caused a significant clinical event, 2) it was associated with a hemoglobin decrease of ≥2 g/dL or transfusion of 2 or more units of blood products, 3) it resulted in reoperation due to bleeding, or 4) it involved retroperitoneal or intracranial hemorrhage.
4 Includes two treated patients who did not undergo a surgical procedure.
5 Occurred at a rate of at least 2% in the group treated with FRAGMIN 5000 IU once daily.
Six of the patients treated with FRAGMIN experienced seven major bleeding reactions. Two of the reactions were wound hematoma (one requiring reoperation), three were bleeding from the operative site, one was intraoperative bleeding due to vessel damage, and one was gastrointestinal bleeding. None of the patients experienced retroperitoneal or intracranial hemorrhage or died of bleeding complications.
In the third hip replacement surgery clinical trial, the incidence of major bleeding reactions was similar in all three treatment groups: 3.6% (18/496) for patients who started FRAGMIN before surgery; 2.5% (12/487) for patients who started FRAGMIN after surgery; and 3.1% (15/489) for patients treated with warfarin sodium.
Abdominal Surgery
Table 7 summarizes bleeding reactions that occurred in clinical trials which studied FRAGMIN 2500 and 5000 IU administered once daily to abdominal surgery patients.
| Table 7 | ||||
| Bleeding Reactions Following Abdominal Surgery | ||||
| Indication | FRAGMIN vs Placebo | FRAGMIN vs FRAGMIN | ||
| Dosing Regimen | Dosing Regimen | |||
| Abdominal Surgery | FRAGMIN 2500 IU once daily subcutaneous n (%) | Placebo once daily subcutaneous n (%) | FRAGMIN 2500 IU once daily subcutaneous n (%) | FRAGMIN 5000 IU once daily subcutaneous n (%) |
| Postoperative Transfusions | 14/182 (7.7) | 13/182 (7.1) | 89/1025 (8.7) | 125/1033 (12.1) |
| Wound Hematoma | 2/79 (2.5) | 2/77 (2.6) | 1/1030 (0.1) | 4/1039 (0.4) |
| Reoperation Due to Bleeding | 1/79 (1.3) | 1/78 (1.3) | 2/1030 (0.2) | 13/1038 (1.3) |
| Injection Site Hematoma | 8/172 (4.7) | 2/174 (1.1) | 36/1026 (3.5) | 57/1035 (5.5) |
| Indication | FRAGMIN vs Heparin | |||
| Dosing Regimen | ||||
| Abdominal Surgery | FRAGMIN 2500 IU once daily subcutaneous n (%) | Heparin 5000 U twice daily subcutaneous n (%) | FRAGMIN 5000 IU once daily subcutaneous n (%) | Heparin 5000 U twice daily subcutaneous n (%) |
| Postoperative Transfusions | 26/459 (5.7) | 36/454 (7.9) | 81/508 (15.9) | 63/498 (12.7) |
| Wound Hematoma | 16/467 (3.4) | 18/467 (3.9) | 12/508 (2.4) | 6/498 (1.2) |
| Reoperation Due to Bleeding | 2/392 (0.5) | 3/392 (0.8) | 4/508 (0.8) | 2/498 (0.4) |
| Injection Site Hematoma | 1/466 (0.2) | 5/464 (1.1) | 36/506 (7.1) | 47/493 (9.5) |
In a trial comparing FRAGMIN 5000 IU once daily to FRAGMIN 2500 IU once daily in patients undergoing surgery for malignancy, the incidence of bleeding reactions was 4.6% and 3.6%, respectively (n.s.). In a trial comparing FRAGMIN 5000 IU once daily to heparin 5000 U twice daily, in the malignancy subgroup the incidence of bleeding reactions was 3.2% and 2.7%, respectively for FRAGMIN and Heparin (n.s.).
Medical Patients with Severely Restricted Mobility During Acute Illness
Table 8 summarizes major bleeding reactions that occurred in a clinical trial of medical patients with severely restricted mobility during acute illness.
| Table 8 | ||
| Bleeding Reactions in Medical Patients with Severely Restricted Mobility During Acute Illness | ||
| Indication | Dosing Regimen | |
| Medical Patients with Severely Restricted Mobility | FRAGMIN
5000 IU once daily subcutaneous n (%) | Placebo
once daily subcutaneous n (%) |
| Major Bleeding Reactions1 at Day 14 | 8/1848 (0.4) | 0/1833 (0) |
| Major Bleeding Reactions1 at Day 21 | 9/1848 (0.5) | 3/1833 (0.2) |
1 A bleeding event was considered major if: 1) it was accompanied by a decrease in hemoglobin of ≥2 g/dL in connection with clinical symptoms; 2) intraocular, spinal/epidural, intracranial, or retroperitoneal bleeding; 3) required transfusion of ≥ 2 units of blood products; 4) required significant medical or surgical intervention; or 5) led to death.
Three of the major bleeding reactions that occurred by Day 21 were fatal, all due to gastrointestinal hemorrhage (two patients in the group treated with FRAGMIN and one in the group receiving placebo).
Patients with Cancer and Acute Symptomatic Venous Thromboembolism
Table 9 summarizes the number of patients with bleeding reactions that occurred in the clinical trial of patients with cancer and acute symptomatic venous thromboembolism. A bleeding event was considered major if it: 1) was accompanied by a decrease in hemoglobin of ≥ 2 g/dL in connection with clinical symptoms; 2) occurred at a critical site (intraocular, spinal/epidural, intracranial, retroperitoneal, or pericardial bleeding); 3) required transfusion of ≥ 2 units of blood products; or 4) led to death. Minor bleeding was classified as clinically overt bleeding that did not meet criteria for major bleeding.
At the end of the six-month study, a total of 46 (13.6%) patients in the FRAGMIN arm and 62 (18.5%) patients in the OAC arm experienced any bleeding event. One bleeding event (hemoptysis in a patient in the FRAGMIN arm at Day 71) was fatal.
| Table 9 | ||||||
| Bleeding Reactions (Major and Any) (As treated population)1 | ||||||
| Study period | FRAGMIN 200 IU/kg (max. 18,000 IU) subcutaneous once daily x 1 month, then 150 IU/kg (max. 18,000 IU) subcutaneous once daily x 5 months | OAC FRAGMIN 200 IU/kg (max 18,000 IU) subcutaneous once daily x 5-7 days and OAC for 6 months (target INR 2-3) |
||||
| Number at risk | Patients with Major Bleeding n (%) | Patients with Any Bleeding n (%) | Number at risk | Patients with Major Bleeding n (%) | Patients with Any Bleeding n (%) |
|
| Total during study | 338 | 19 (5.6) | 46 (13.6) | 335 | 12 (3.6) | 62 (18.5) |
| Week 1 | 338 | 4 (1.2) | 15 (4.4) | 335 | 4 (1.2) | 12 (3.6) |
| Weeks 2-4 | 332 | 9 (2.7) | 17 (5.1) | 321 | 1 (0.3) | 12 (3.7) |
| Weeks 5-28 | 297 | 9 (3.0) | 26 (8.8) | 267 | 8 (3.0) | 40 (15.0) |
1 Patients with multiple bleeding episodes within any time interval were counted only once in that interval. However, patients with multiple bleeding episodes that occurred at different time intervals were counted once in each interval in which the event occurred.
Thrombocytopenia
[see Warnings and Precautions (5.2)]
Elevations of Serum Transaminases
In FRAGMIN clinical trials supporting non-cancer indications, where hepatic transaminases were measured, asymptomatic increases in transaminase levels (SGOT/AST and SGPT/ALT) greater than three times the upper limit of normal of the laboratory reference range were seen in 4.7% and 4.2%, respectively, of patients during treatment with FRAGMIN.
In the FRAGMIN clinical trial of patients with cancer and acute symptomatic venous thromboembolism treated with FRAGMIN for up to 6 months, asymptomatic increases in transaminase levels, AST and ALT, greater than three times the upper limit of normal of the laboratory reference range were reported in 8.9% and 9.5% of patients, respectively. The frequencies of Grades 3 and 4 increases in AST and ALT, as classified by the National Cancer Institute, Common Toxicity Criteria (NCI-CTC) Scoring System, were 3% and 3.8%, respectively. Grades 2, 3 & 4 combined have been reported in 12% and 14% of patients, respectively.
Other
Allergic Reactions: Allergic reactions (i.e., pruritus, rash, fever, injection site reaction, bullous eruption) have occurred. Cases of anaphylactoid reactions have been reported.
Local Reactions: Pain at the injection site, the only non-bleeding event determined to be possibly or probably related to treatment with FRAGMIN and reported at a rate of at least 2% in the group treated with FRAGMIN, was reported in 4.5% of patients treated with FRAGMIN 5000 IU once daily vs 11.8% of patients treated with heparin 5000 U twice daily in the abdominal surgery trials. In the hip replacement trials, pain at injection site was reported in 12% of patients treated with FRAGMIN 5000 IU once daily vs 13% of patients treated with heparin 5000 U three times a day.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during postapproval use of FRAGMIN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Since first international market introduction in 1985, there have been more than 15 reports of epidural or spinal hematoma formation with concurrent use of dalteparin sodium and spinal/epidural anesthesia or spinal puncture. The majority of patients had postoperative indwelling epidural catheters placed for analgesia or received additional drugs affecting hemostasis. In some cases the hematoma resulted in long-term or permanent paralysis (partial or complete) [see Boxed Warning].
Skin necrosis has occurred. There have been cases of alopecia reported that improved on drug discontinuation.
7 DRUG INTERACTIONS
Use FRAGMIN with care in patients receiving oral anticoagulants, platelet inhibitors, and thrombolytic agents because of increased risk of bleeding [see Warning and Precautions (5)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
There are no adequate and well-controlled studies of FRAGMIN use in pregnant women. In reproductive and developmental toxicity studies, pregnant rats and rabbits received dalteparin sodium at intravenous doses up to 2400 IU/kg (14,160 IU/m2) (rats) and 4800 IU/kg (40,800 IU/m2) (rabbits). These exposures were 2 to 4 times (rats) and 4 times (rabbits) the human dose of 100 IU/kg dalteparin based on the body surface area. No evidence of impaired fertility or harm to the fetuses occurred in these studies. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Cases of "Gasping Syndrome" have occurred in premature infants when large amounts of benzyl alcohol have been administered (99-404 mg/kg/day). The 9.5 mL and the 3.8 mL multiple-dose vials of FRAGMIN contain 14 mg/mL of benzyl alcohol [see Warnings and Precautions (5.3)].
8.3 Nursing Mothers
Based on limited published data dalteparin is minimally excreted in human milk. One study of 15 lactating women receiving prophylactic doses of dalteparin, in the immediate postpartum period, detected small amounts of anti-Xa activity (range < 0.005 to 0.037 IU/ml) in breast milk that were equivalent to a milk/plasma ratio of < 0.025-0.224. Oral absorption of LMWH is extremely low, but the clinical implications, if any, of this small amount of anticoagulant activity on a nursing infant are unknown. Caution should be exercised when FRAGMIN is administered to a nursing woman.
8.5 Geriatric Use
Of the total number of patients in clinical studies of FRAGMIN, 5516 patients were 65 years of age or older and 2237 were 75 or older. No overall differences in effectiveness were observed between these subjects and younger subjects. Some studies suggest that the risk of bleeding increases with age. Postmarketing surveillance and literature reports have not revealed additional differences in the safety of FRAGMIN between elderly and younger patients. Give careful attention to dosing intervals and concomitant medications (especially antiplatelet medications) in geriatric patients, particularly in those with low body weight (< 45 kg) and those predisposed to decreased renal function [see Warnings and Precautions (5) and Clinical Pharmacology (12)].
10 OVERDOSAGE
An excessive dosage of FRAGMIN Injection may lead to hemorrhagic complications. These may generally be stopped by slow intravenous injection of protamine sulfate (1% solution), at a dose of 1 mg protamine for every 100 anti-Xa IU of FRAGMIN given. If the APTT measured 2 to 4 hours after the first infusion remains prolonged, a second infusion of 0.5 mg protamine sulfate per 100 anti-Xa IU of FRAGMIN may be administered. Even with these additional doses of protamine, the APTT may remain more prolonged than would usually be found following administration of unfractionated heparin. In all cases, the anti-Factor Xa activity is never completely neutralized (maximum about 60 to 75%).
Take particular care to avoid overdosage with protamine sulfate. Administration of protamine sulfate can cause severe hypotensive and anaphylactoid reactions. Because fatal reactions, often resembling anaphylaxis, have been reported with protamine sulfate, give protamine sulfate only when resuscitation techniques and treatment for anaphylactic shock are readily available. For additional information, consult the labeling of Protamine Sulfate Injection, USP, products.
11 DESCRIPTION
FRAGMIN Injection (dalteparin sodium injection) is a sterile, low molecular weight heparin. It is available in single-dose, prefilled syringes preassembled with a needle guard device, and multiple-dose vials. With reference to the W.H.O. First International Low Molecular Weight Heparin Reference Standard, each syringe contains either 2500, 5000, 7500, 10,000, 12,500, 15,000 or 18,000 anti-Factor Xa international units (IU), equivalent to 16, 32, 48, 64, 80, 96 or 115.2 mg dalteparin sodium, respectively. Each multiple-dose vial contains either 10,000 or 25,000 anti-Factor Xa IU per 1 mL (equivalent to 64 or 160 mg dalteparin sodium, respectively), for a total of 95,000 anti-Factor Xa IU per vial.
Each prefilled syringe also contains Water for Injection and sodium chloride, when required, to maintain physiologic ionic strength. The prefilled syringes are preservative-free. Each multiple-dose vial also contains Water for Injection and 14 mg of benzyl alcohol per mL as a preservative. The pH of both formulations is 5.0 to 7.5. [See Dosage and Administration (2) and How Supplied (16)]
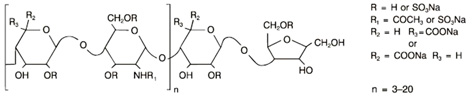
Dalteparin sodium is produced through controlled nitrous acid depolymerization of sodium heparin from porcine intestinal mucosa followed by a chromatographic purification process. It is composed of strongly acidic sulfated polysaccharide chains (oligosaccharide, containing 2,5-anhydro-D-mannitol residues as end groups) with an average molecular weight of 5000 and about 90% of the material within the range 2000-9000. The molecular weight distribution is:
< 3000 daltons 3.0-15%
3000 to 8000 daltons 65.0-78.0%
> 8000 daltons 14.0-26.0%
STRUCTURAL FORMULA
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Dalteparin is a low molecular weight heparin with antithrombotic properties. It acts by enhancing the inhibition of Factor Xa and thrombin by antithrombin. In humans, dalteparin potentiates preferentially the inhibition of coagulation Factor Xa, while only slightly affecting the activated partial thromboplastin time (APTT).
12.2 Pharmacodynamics
Doses of FRAGMIN Injection of up to 10,000 anti-Factor Xa IU administered subcutaneously as a single dose or two 5000 IU doses 12 hours apart to healthy subjects did not produce a significant change in platelet aggregation, fibrinolysis, or global clotting tests such as prothrombin time (PT), thrombin time (TT) or APTT. Subcutaneous administration of doses of 5000 IU twice daily of FRAGMIN for seven consecutive days to patients undergoing abdominal surgery did not markedly affect APTT, Platelet Factor 4 (PF4), or lipoprotein lipase.
12.3 Pharmacokinetics
Mean peak levels of plasma anti-Factor Xa activity following single subcutaneous doses of 2500, 5000 and 10,000 IU were 0.19 ± 0.04, 0.41 ± 0.07 and 0.82 ± 0.10 IU/mL, respectively, and were attained in about 4 hours in most subjects. Absolute bioavailability in healthy volunteers, measured as the anti-Factor Xa activity, was 87 ± 6%. Increasing the dose from 2500 to 10,000 IU resulted in an overall increase in anti-Factor Xa AUC that was greater than proportional by about one-third.
Peak anti-Factor Xa activity increased more or less linearly with dose over the same dose range. There appeared to be no appreciable accumulation of anti-Factor Xa activity with twice-daily dosing of 100 IU/kg subcutaneously for up to 7 days.
The volume of distribution for dalteparin anti-Factor Xa activity was 40 to 60 mL/kg. The mean plasma clearances of dalteparin anti-Factor Xa activity in normal volunteers following single intravenous bolus doses of 30 and 120 anti-Factor Xa IU/kg were 24.6 ± 5.4 and 15.6 ± 2.4 mL/hr/kg, respectively. The corresponding mean disposition half-lives were 1.47 ± 0.3 and 2.5 ± 0.3 hours.
Following intravenous doses of 40 and 60 IU/kg, mean terminal half-lives were 2.1 ± 0.3 and 2.3 ± 0.4 hours, respectively. Longer apparent terminal half-lives (3 to 5 hours) are observed following subcutaneous dosing, possibly due to delayed absorption. In patients with chronic renal insufficiency requiring hemodialysis, the mean terminal half-life of anti-Factor Xa activity following a single intravenous dose of 5000 IU FRAGMIN was 5.7 ± 2.0 hours, i.e. considerably longer than values observed in healthy volunteers, therefore, greater accumulation can be expected in these patients.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Dalteparin sodium has not been tested for its carcinogenic potential in long-term animal studies. It was not mutagenic in the in vitro Ames Test, mouse lymphoma cell forward mutation test and human lymphocyte chromosomal aberration test and in the in vivo mouse micronucleus test. Dalteparin sodium at subcutaneous doses up to 1200 IU/kg (7080 IU/m2) did not affect the fertility or reproductive performance of male and female rats.
14 CLINICAL STUDIES
14.1 Prophylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction
In a double-blind, randomized, placebo-controlled clinical trial, patients who recently experienced unstable angina with EKG changes or non-Q-wave myocardial infarction (MI) were randomized to FRAGMIN Injection 120 IU/kg or placebo every 12 hours subcutaneously. In this trial, unstable angina was defined to include only angina with EKG changes. All patients, except when contraindicated, were treated concurrently with aspirin (75 mg once daily) and beta blockers. Treatment was initiated within 72 hours of the event (the majority of patients received treatment within 24 hours) and continued for 5 to 8 days. A total of 1506 patients were enrolled and treated; 746 received FRAGMIN and 760 received placebo. The mean age of the study population was 68 years (range 40 to 90 years) and the majority of patients were white (99.7%) and male (63.9%). The combined incidence of the endpoint of death or myocardial infarction was lower for FRAGMIN compared with placebo at 6 days after initiation of therapy. These results were observed in an analysis of all-randomized and all-treated patients. The combined incidence of death, MI, need for intravenous heparin or intravenous. nitroglycerin, and revascularization was also lower for FRAGMIN than for placebo (see Table 10).
| Table 10 | ||
| Efficacy of FRAGMIN in the Prophylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction | ||
| Indication | Dosing Regimen | |
| FRAGMIN
120 IU/kg/every 12 hr subcutaneous n (%) | Placebo
every 12 hr subcutaneous n (%) |
|
| All Treated Unstable Angina and Non-Q-Wave MI Patients | 746 | 760 |
| Primary Endpoints - 6 day timepoint Death, MI | 13/741 (1.8)1 | 36/757 (4.8) |
| Secondary Endpoints - 6 day timepoint Death, MI, intravenous heparin, i.v. nitroglycerin, Revascularization | 59/739 (8.0)1 | 106/756 (14.0) |
1 p-value = 0.001
In a second randomized, controlled trial designed to evaluate long-term treatment with FRAGMIN (days 6 to 45), data were also collected comparing 1-week (5 to 8 days) treatment of FRAGMIN 120 IU/kg every 12 hours subcutaneously with heparin at an APTT-adjusted dosage. All patients, except when contraindicated, were treated concurrently with aspirin (100 to 165 mg per day). Of the 1499 patients enrolled, 1482 patients were treated; 751 received FRAGMIN and 731 received heparin. The mean age of the study population was 64 years (range 25 to 92 years) and the majority of patients were white (96.0%) and male (64.2%). The incidence of the combined endpoint of death, myocardial infarction, or recurrent angina during this 1-week treatment period (5 to 8 days) was 9.3% for FRAGMIN and 7.6% for heparin (p=0.323).
14.2 Prophylaxis of Deep Vein Thrombosis in Patients Following Hip Replacement Surgery
In an open-label randomized study, FRAGMIN 5000 IU administered once daily subcutaneously was compared with warfarin sodium, administered orally, in patients undergoing hip replacement surgery. Treatment with FRAGMIN was initiated with a 2500 IU dose subcutaneously within 2 hours before surgery, followed by a 2500 IU dose subcutaneously the evening of the day of surgery. Then, a dosing regimen of FRAGMIN 5000 IU subcutaneously once daily was initiated on the first postoperative day. The first dose of warfarin sodium was given the evening before surgery, then continued daily at a dose adjusted for INR 2 to 3. Treatment in both groups was then continued for 5 to 9 days postoperatively. Of the 580 patients enrolled, 553 were treated and 550 underwent surgery. Of those who underwent surgery, 271 received FRAGMIN and 279 received warfarin sodium. The mean age of the study population was 63 years (range 20 to 92 years) and the majority of patients were white (91.1%) and female (52.9%). The incidence of deep vein thrombosis (DVT), as determined by evaluable venography, was significantly lower for the group treated with FRAGMIN compared with patients treated with warfarin sodium (see Table 11).
| Table 11 | ||
| Efficacy of FRAGMIN in the Prophylaxis of Deep Vein Thrombosis Following Hip Replacement Surgery |
||
| Indication | Dosing Regimen | |
| FRAGMIN
5000 IU once daily1 subcutaneous n (%) | Warfarin Sodium
once daily2 oral n (%) |
|
| All Treated Hip Replacement Surgery Patients | 271 | 279 |
| Treatment Failures in Evaluable Patients DVT, Total | 28/192 (14.6)3 | 49/190 (25.8) |
| Proximal DVT | 10/192 (5.2)4 | 16/190 (8.4) |
| PE | 2/271 (0.7) | 2/279 (0.7) |
1 The daily dose on the day of surgery was divided: 2500 IU was given two hours before surgery and again in the evening of the day of surgery.
2 Warfarin sodium dosage was adjusted to maintain a prothrombin time index of 1.4 to 1.5, corresponding to an International Normalized Ratio (INR) of approximately 2.5
3 p-value = 0.006
4 p-value = 0.185
In a second single-center, double-blind study of patients undergoing hip replacement surgery, FRAGMIN 5000 IU once daily subcutaneously starting the evening before surgery, was compared with heparin 5000 U subcutaneously three times a day, starting the morning of surgery. Treatment in both groups was continued for up to 9 days postoperatively. Of the 140 patients enrolled, 139 were treated and 136 underwent surgery. Of those who underwent surgery, 67 received FRAGMIN and 69 received heparin. The mean age of the study population was 69 years (range 42 to 87 years) and the majority of patients were female (58.8%). In the intent-to-treat analysis, the incidence of proximal DVT was significantly lower for patients treated with FRAGMIN compared with patients treated with heparin (6/67 vs 18/69; p=0.012). The incidence of pulmonary embolism detected by lung scan was also significantly lower in the group treated with FRAGMIN (9/67 vs 19/69; p=0.032).
A third multi-center, double-blind, randomized study evaluated a postoperative dosing regimen of FRAGMIN for thromboprophylaxis following total hip replacement surgery. Patients received either FRAGMIN or warfarin sodium, randomized into one of three treatment groups. One group of patients received the first dose of FRAGMIN 2500 IU subcutaneous within 2 hours before surgery, followed by another dose of FRAGMIN 2500 IU subcutaneous at least 4 hours (6.6 ± 2.3 hr) after surgery. Another group received the first dose of FRAGMIN 2500 IU subcutaneous at least 4 hours (6.6 ± 2.4 hr) after surgery. Then, both of these groups began a dosing regimen of FRAGMIN 5000 IU once daily subcutaneous on postoperative day 1. The third group of patients received warfarin sodium the evening of the day of surgery, then continued daily at a dose adjusted to maintain INR 2 to 3. Treatment for all groups was continued for 4 to 8 days postoperatively, after which time all patients underwent bilateral venography.
In the total enrolled study population of 1501 patients, 1472 patients were treated; 496 received FRAGMIN (first dose before surgery), 487 received FRAGMIN (first dose after surgery) and 489 received warfarin sodium. The mean age of the study population was 63 years (range 18 to 91 years) and the majority of patients were white (94.4%) and female (51.8%).
Administration of the first dose of FRAGMIN after surgery was as effective in reducing the incidence of thromboembolic reactions as administration of the first dose of FRAGMIN before surgery (44/336 vs 37/338; p=0.448). Both dosing regimens of FRAGMIN were more effective than warfarin sodium in reducing the incidence of thromboembolic reactions following hip replacement surgery.
14.3 Prophylaxis of Deep Vein Thrombosis Following Abdominal Surgery in Patients at Risk for Thromboembolic Complications
Abdominal surgery patients at risk include those who are over 40 years of age, obese, undergoing surgery under general anesthesia lasting longer than 30 minutes, or who have additional risk factors such as malignancy or a history of deep vein thrombosis or pulmonary embolism.
FRAGMIN administered once daily subcutaneously beginning prior to surgery and continued for 5 to 10 days after surgery, reduced the risk of DVT in patients at risk for thromboembolic complications in two double-blind, randomized, controlled clinical trials performed in patients undergoing major abdominal surgery. In the first study, a total of 204 patients were enrolled and treated; 102 received FRAGMIN and 102 received placebo. The mean age of the study population was 64 years (range 40 to 98 years) and the majority of patients were female (54.9%). In the second study, a total of 391 patients were enrolled and treated; 195 received FRAGMIN and 196 received heparin. The mean age of the study population was 59 years (range 30 to 88 years) and the majority of patients were female (51.9%). FRAGMIN 2500 IU was superior to placebo and similar to heparin in reducing the risk of DVT (see Tables 12 and 13).
| Table 12 | ||
| Efficacy of FRAGMIN in the Prophylaxis of Deep Vein Thrombosis Following Abdominal Surgery | ||
| Indication | Dosing Regimen | |
| FRAGMIN
2500 IU once daily subcutaneous n (%) | Placebo
once daily subcutaneous n (%) |
|
| All Treated Abdominal Surgery Patients | 102 | 102 |
| Treatment Failures in Evaluable Patients Total Thromboembolic Reactions | 4/91 (4.4)1 | 16/91 (17.6) |
| Proximal DVT | 0 | 5/91 (5.5) |
| Distal DVT | 4/91 (4.4) | 11/91 (12.1) |
| PE | 0 | 2/91 (2.2)2 |
1 p-value = 0.008
2 Both patients also had DVT, 1 proximal and 1 distal
| Table 13 | ||
| Efficacy of FRAGMIN in the Prophylaxis of Deep Vein Thrombosis Following Abdominal Surgery |
||
| Indication | Dosing Regimen | |
| FRAGMIN
2500 IU once daily subcutaneous n (%) | Heparin
5000 U twice daily subcutaneous n (%) |
|
| All Treated Abdominal Surgery Patients | 195 | 196 |
| Treatment Failures in Evaluable Patients Total Thromboembolic Reactions | 7/178 (3.9)1 | 7/174 (4.0) |
| Proximal DVT | 3/178 (1.7) | 4/174 (2.3) |
| Distal DVT | 3/178 (1.7) | 3/174 (1.7) |
| PE | 1/178 (0.6) | 0 |
1 p-value = 0.74
In a third double-blind, randomized study performed in patients undergoing major abdominal surgery with malignancy, FRAGMIN 5000 IU subcutaneous once daily was compared with FRAGMIN 2500 IU subcutaneous once daily. Treatment was continued for 6 to 8 days. A total of 1375 patients were enrolled and treated; 679 received FRAGMIN 5000 IU and 696 received 2500 IU. The mean age of the combined groups was 71 years (range 40 to 95 years). The majority of patients were female (51.0%). FRAGMIN 5000 IU once daily was more effective than FRAGMIN 2500 IU once daily in reducing the risk of DVT in patients undergoing abdominal surgery with malignancy (see Table 14).
| Table 14 | ||
| Efficacy of FRAGMIN in the Prophylaxis of Deep Vein Thrombosis Following Abdominal Surgery |
||
| Indication | Dosing Regimen | |
| FRAGMIN
2500 IU once daily subcutaneous n (%) | FRAGMIN
5000 IU once daily subcutaneous n (%) |
|
| All Treated Abdominal Surgery Patients1 | 696 | 679 |
| Treatment Failures in Evaluable Patients Total Thromboembolic Reactions | 99/656 (15.1)2 | 60/645 (9.3) |
| Proximal DVT | 18/657 (2.7) | 14/646 (2.2) |
| Distal DVT | 80/657 (12.2) | 41/646 (6.3) |
| PE | ||
| Fatal | 1/674 (0.1) | 1/669 (0.1) |
| Non-fatal | 2 | 4 |
1 Major abdominal surgery with malignancy
2 p-value = 0.001
14.4 Prophylaxis of Deep Vein Thrombosis in Medical Patients at Risk for Thromboembolic Complications Due to Severely Restricted Mobility During Acute Illness
In a double-blind, multi-center, randomized, placebo-controlled clinical trial, general medical patients with severely restricted mobility who were at risk of venous thromboembolism were randomized to receive either FRAGMIN 5000 IU or placebo subcutaneously once daily during Days 1 to 14 of the study. These patients had an acute medical condition requiring a projected hospital stay of at least 4 days, and were confined to bed during waking hours. The study included patients with congestive heart failure (NYHA Class III or IV), acute respiratory failure not requiring ventilatory support, and the following acute conditions with at least one risk factor occurring in > 1% of treated patients: acute infection (excluding septic shock), acute rheumatic disorder, acute lumbar or sciatic pain, vertebral compression, or acute arthritis of the lower extremities. Risk factors include > 75 years of age, cancer, previous DVT/PE, obesity and chronic venous insufficiency. A total of 3681 patients were enrolled and treated: 1848 received FRAGMIN and 1833 received placebo. The mean age of the study population was 69 years (range 26 to 99 years), 92.1% were white and 51.9% were female. The primary efficacy endpoint was evaluated at Day 21 and was defined as at least one of the following within Days 1 to 21 of the study: asymptomatic DVT (diagnosed by compression ultrasound), a confirmed symptomatic DVT, a confirmed pulmonary embolism or sudden death. The follow-up extended through Day 90.
When given at a dose of 5000 IU once a day subcutaneously, FRAGMIN significantly reduced the incidence of thromboembolic reactions including verified DVT by Day 21 (see Table 15). The prophylactic effect was sustained through Day 90.
| Table 15 | ||
| Efficacy of FRAGMIN in the Prophylaxis of Deep Vein Thrombosis in Medical Patients with Severely Restricted Mobility During Acute Illness | ||
| Indication | Dosing Regimen | |
| FRAGMIN
5000 IU once daily subcutaneous n (%) | Placebo
once daily subcutaneous n (%) |
|
| All Treated Medical Patients During Acute Illness | 1848 | 1833 |
| Treatment failure in evaluable patients (Day 21)1
DVT, PE, or sudden death | 42/1518 (2.8)2 | 73/1473 (5.0) |
| Total Thromboembolic Reactions (Day 21) | 37/1513 (2.5) | 70/1470 (4.8) |
| Total DVT | 32/1508 (2.1) | 64/1464 (4.4) |
| Proximal DVT | 29/1518 (1.9) | 60/1474 (4.1) |
| Symptomatic VTE | 10/1759 (0.6) | 17/1740 (1.0) |
| PE | 5/1759 (0.3) | 6/1740 (0.3) |
| Sudden Death | 5/1829 (0.3) | 3/1807 (0.2) |
1 Defined as DVT (diagnosed by compression ultrasound at Day 21 + 3), confirmed symptomatic DVT, confirmed PE or sudden death.
2 p-value = 0.0015
14.5 Patients with Cancer and Acute Symptomatic Venous Thromboembolism
In a prospective, multi-center, open-label, clinical trial, 676 patients with cancer and newly diagnosed, objectively confirmed acute deep vein thrombosis (DVT) and/or pulmonary embolism (PE) were studied. Patients were randomized to either FRAGMIN 200 IU/kg subcutaneous (max 18,000 IU subcutaneous daily for one month) then 150 IU/kg subcutaneous (max 18,000 IU subcutaneous daily for five months (FRAGMIN arm) or FRAGMIN 200 IU/kg subcutaneous (max 18,000 IU subcutaneous daily for five to seven days and oral anticoagulant for six months (OAC arm). In the OAC arm, oral anticoagulation was adjusted to maintain an INR of 2 to 3. Patients were evaluated for recurrence of symptomatic venous thromboembolism (VTE) every two weeks for six months.
The median age of patients was 64 years (range: 22 to 89 years); 51.5% of patients were females; 95.3% of patients were Caucasians. Types of tumors were: gastrointestinal tract (23.7%), genito-urinary (21.5%), breast (16%), lung (13.3%), hematological tumors (10.4%) and other tumors (15.1%).
A total of 27 (8.0%) and 53 (15.7%) patients in the FRAGMIN and OAC arms, respectively, experienced at least one episode of an objectively confirmed, symptomatic DVT and/or PE during the 6-month study period. Most of the difference occurred during the first month of treatment (see Table 16). The benefit was maintained over the 6-month study period.
| Table 16 | ||||||
| Recurrent VTE in Patients with Cancer (Intention to treat population)1 | ||||||
| Study Period | FRAGMIN arm | OAC arm | ||||
| FRAGMIN 200 IU/kg (max. 18,000 IU) subcutaneous once daily x 1 month, then 150 IU/kg (max. 18,000 IU) subcutaneous once daily x 5 months | FRAGMIN 200 IU/kg (max 18,000 IU) subcutaneous once daily x 5-7 days and OAC for 6 months (target INR 2-3) | |||||
| Number at Risk | Patients with VTE | % | Number at Risk | Patients with VTE | % | |
| Total | 338 | 27 | 8.0 | 338 | 53 | 15.7 |
| Week 1 | 338 | 5 | 1.5 | 338 | 8 | 2.4 |
| Weeks 2-4 | 331 | 6 | 1.8 | 327 | 25 | 7.6 |
| Weeks 5-28 | 307 | 16 | 5.2 | 284 | 20 | 7.0 |
1 Three patients in the FRAGMIN arm and 5 patients in the OAC arm experienced more than 1 VTE over the 6-month study period.
In the intent-to-treat population that included all randomized patients, the primary comparison of the cumulative probability of the first VTE recurrence over the 6-month study period was statistically significant (p < 0.01) in favor of the FRAGMIN arm, with most of the treatment difference evident in the first month.
16 HOW SUPPLIED/STORAGE AND HANDLING
After first penetration of the rubber stopper, store the multiple-dose vials at room temperature for up to 2 weeks.
| Dosage Form | Strength | Package Size | NDC Number |
| Single-dose prefilled syringe1 | 2,500 IU / 0.2 mL | 10 Syringes | 62856-250-10 |
| 5,000 IU / 0.2 mL | 10 Syringes | 62856-500-10 | |
| 7,500 IU / 0.3 mL | 10 Syringes | 62856-750-10 | |
| 10,000 IU / 0.4 mL | 10 Syringes | 62856-100-10 | |
| Single-dose graduated syringe2 | 10,000 IU / 1 mL | 10 Syringes | 62856-101-10 |
| Single-dose prefilled syringe1 | 12,500 IU / 0.5 mL | 10 Syringes | 62856-125-10 |
| 15,000 IU / 0.6 mL | 10 Syringes | 62856-150-10 | |
| 18,000 IU / 0.72 mL | 10 Syringes | 62856-180-10 | |
| Multiple dose vial | 95,000 IU / 3.8 mL | 3.8 mL Vial | 62856-251-01 |
| Multiple dose vial | 95,000 IU / 9.5 mL | 9.5 mL Vial | 62856-102-01 |
1 Single-dose prefilled syringe, affixed with a 27-gauge x 1/2 inch needle and preassembled with UltraSafe Passive™ Needle Guard devices.
2 Single-dose graduated syringe, affixed with a 27-gauge x 1/2 inch needle and preassembled with UltraSafe Passive™ Needle Guard devices. UltraSafe Passive™ Needle Guard is a trademark of Safety Syringes, Inc.
Store at controlled room temperature 20° to 25°C (68° to 77°F) [see USP].
17 PATIENT COUNSELING INFORMATION
If patients have had neuraxial anesthesia or spinal puncture, and particularly, if they are taking concomitant NSAIDs, platelet inhibitors, or other anticoagulants, inform the patients to watch for signs and symptoms of spinal or epidural hematoma, such as tingling, numbness (especially in the lower limbs) and muscular weakness. If any of these symptoms occur the patient should contact his or her physician immediately.
Additionally, the use of aspirin and other NSAIDs may enhance the risk of hemorrhage. Discontinue their use prior to dalteparin therapy whenever possible; if co-administration is essential, the patient's clinical and laboratory status should be closely monitored [see Drug Interactions (7)].
Inform patients:
- of the instructions for injecting FRAGMIN if their therapy is to continue after discharge from the hospitals.
- it may take them longer than usual to stop bleeding.
- they may bruise and/or bleed more easily when they are treated with FRAGMIN.
- they should report any unusual bleeding, bruising, or signs of thrombocytopenia (such as a rash of dark red spots under the skin) to their physician [see Warnings and Precautions (5.1, 5.2)].
- to tell their physicians and dentists they are taking FRAGMIN and/or any other product known to affect bleeding before any surgery is scheduled and before any new drug is taken [see Warnings and Precautions (5.1)].
- to tell their physicians and dentists of all medications they are taking, including those obtained without a prescription, such as aspirin or other NSAIDs [see Drug Interactions (7)].
FRAGMIN is a registered trademark of Pfizer Health AB and is licensed to Eisai Inc.
Manufactured for
Eisai Inc.
Woodcliff Lake, NJ 07677
Manufactured by
Pfizer Inc
New York, NY 10017
Made in Belgium
(multiple-dose vials)
Jointly manufactured by
Pfizer Inc, New York, NY 10017
and Vetter Pharma-Fertigung, GmbH & Co. KG
Ravensburg, Germany
(prefilled syringes)
| FRAGMIN
dalteparin sodium injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FRAGMIN
dalteparin sodium injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FRAGMIN
dalteparin sodium injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FRAGMIN
dalteparin sodium injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FRAGMIN
dalteparin sodium injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FRAGMIN
dalteparin sodium injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FRAGMIN
dalteparin sodium injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FRAGMIN
dalteparin sodium injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FRAGMIN
dalteparin sodium injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FRAGMIN
dalteparin sodium injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Eisai Inc. (831600833) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Health AB | 354433591 | API MANUFACTURE(62856-250, 62856-500, 62856-750, 62856-100, 62856-101, 62856-125, 62856-150, 62856-180, 62856-251, 62856-102) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Manufacturing Belgium NV | 370156507 | MANUFACTURE(62856-250, 62856-500, 62856-750, 62856-100, 62856-101, 62856-125, 62856-150, 62856-180, 62856-251, 62856-102) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vetter Pharma-Fertigung GmbH & Co. KG | 312670654 | MANUFACTURE(62856-250, 62856-500, 62856-750, 62856-100, 62856-101, 62856-125, 62856-150, 62856-180, 62856-251, 62856-102) | |