PVP-I POUCH FOIL-FOIL- povidone-iodine solution
Dukal Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
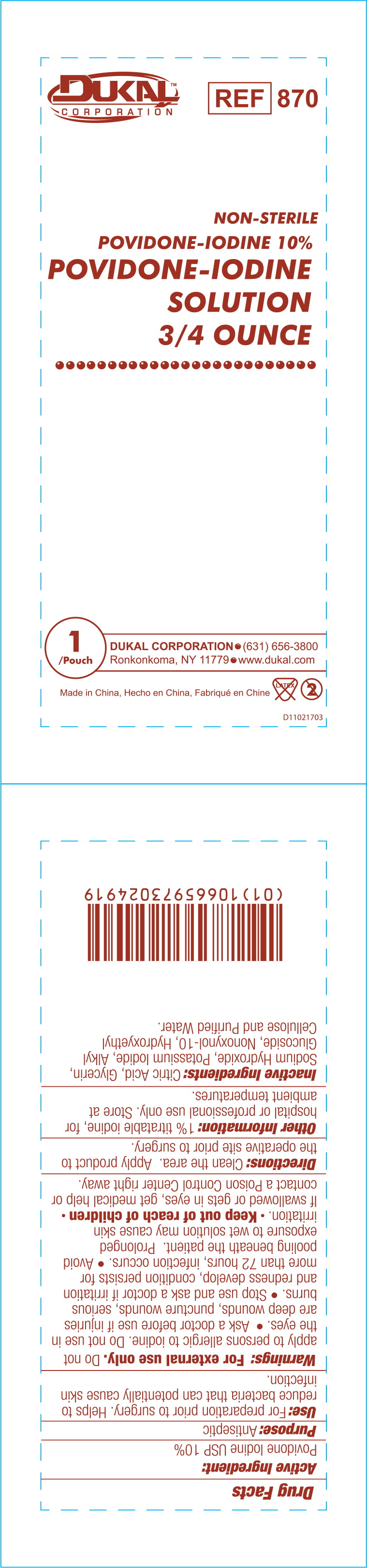
Drug Facts
Use:
For preparation prior to surgery. Helps to reduce bacteria that can potentially cause skin infection.
Warnings:
For external use only.
Do not apply to persons allergic to iodine. Do not use in the eyes.
- Ask a doctor before use if injuries are deep wounds, puncture wounds, serious burns.
Other Information:
1% titratable iodine, for hospital or professional use only. Store at ambient temperatures.
Inactive Ingredients:
Citric Acid, Glycerin, Sodium Hydroxide, Potassium Iodide, Alkyl Glucoside, Nonoxynol-10, Hydroxyethyl Cellulose and Purified Water.
Principal Display Panel - PVP-I 3/4 Ounce Solution Case Label
DUKAL™
CORPORATION
REF 870-500
NON-STERILE POVIDONE-IODINE 10%
POVIDONE-IODINE
SOLUTION 3/4 OUNCE
500/Case
1/Pack
Manufactured For: DUKAL CORPORATION • (631) 656-3800
Ronkonkoma, NY 11779 • www.dukal.com
Made in China, Hecho en China, Fabriqué en Chine
(01) 40665973024910
| PVP-I POUCH FOIL-FOIL
povidone-iodine solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Dukal Corporation (791014871) |