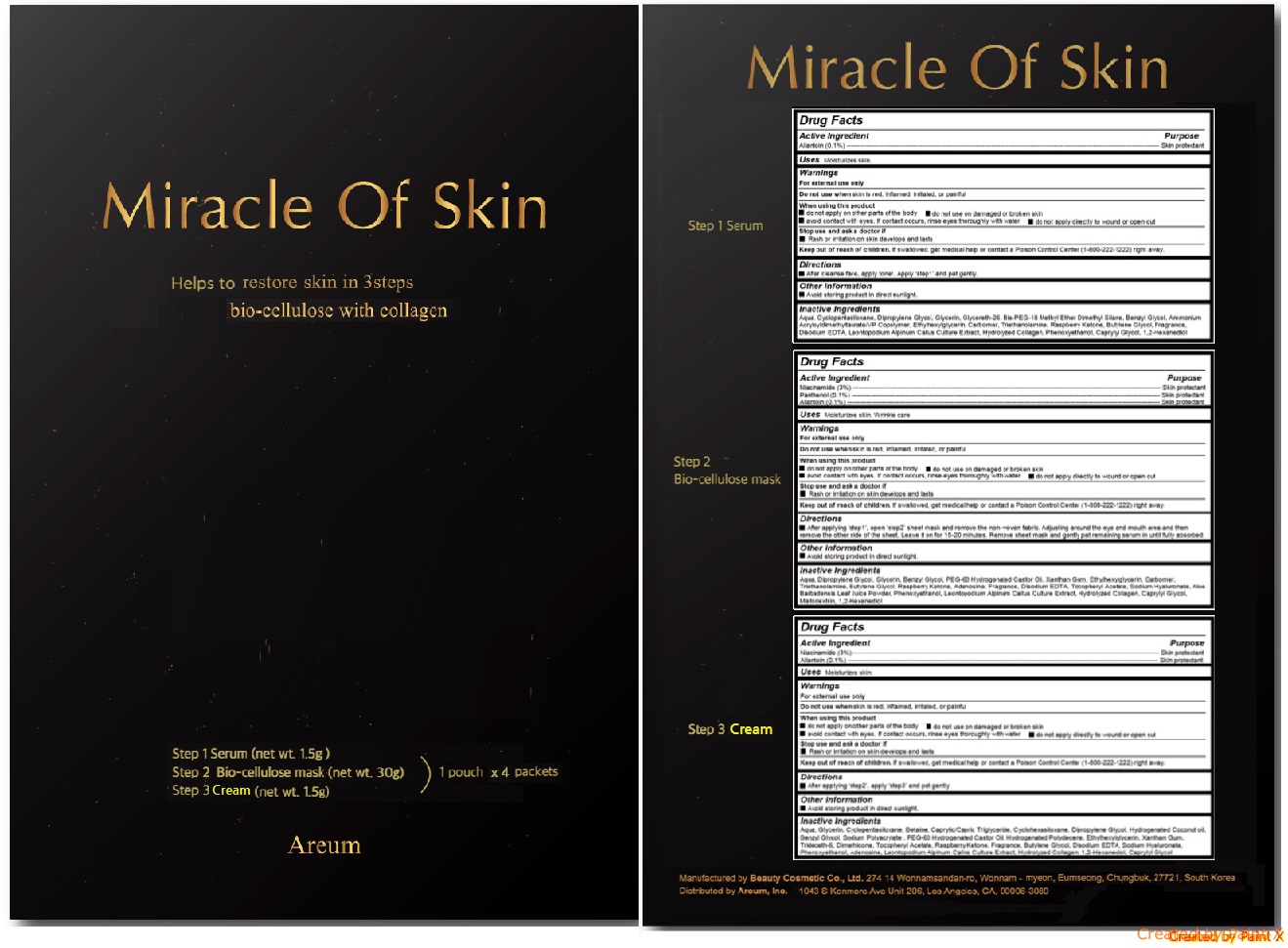
Label: MIRACLE OF SKIN- allantoin, niacinamide, pantheon kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 72148-100-01, 72148-100-02, 72148-100-03, 72148-101-01, view more72148-101-02, 72148-101-03, 72148-102-01, 72148-102-02, 72148-102-03, 72148-103-01 - Packager: Areum, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 23, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
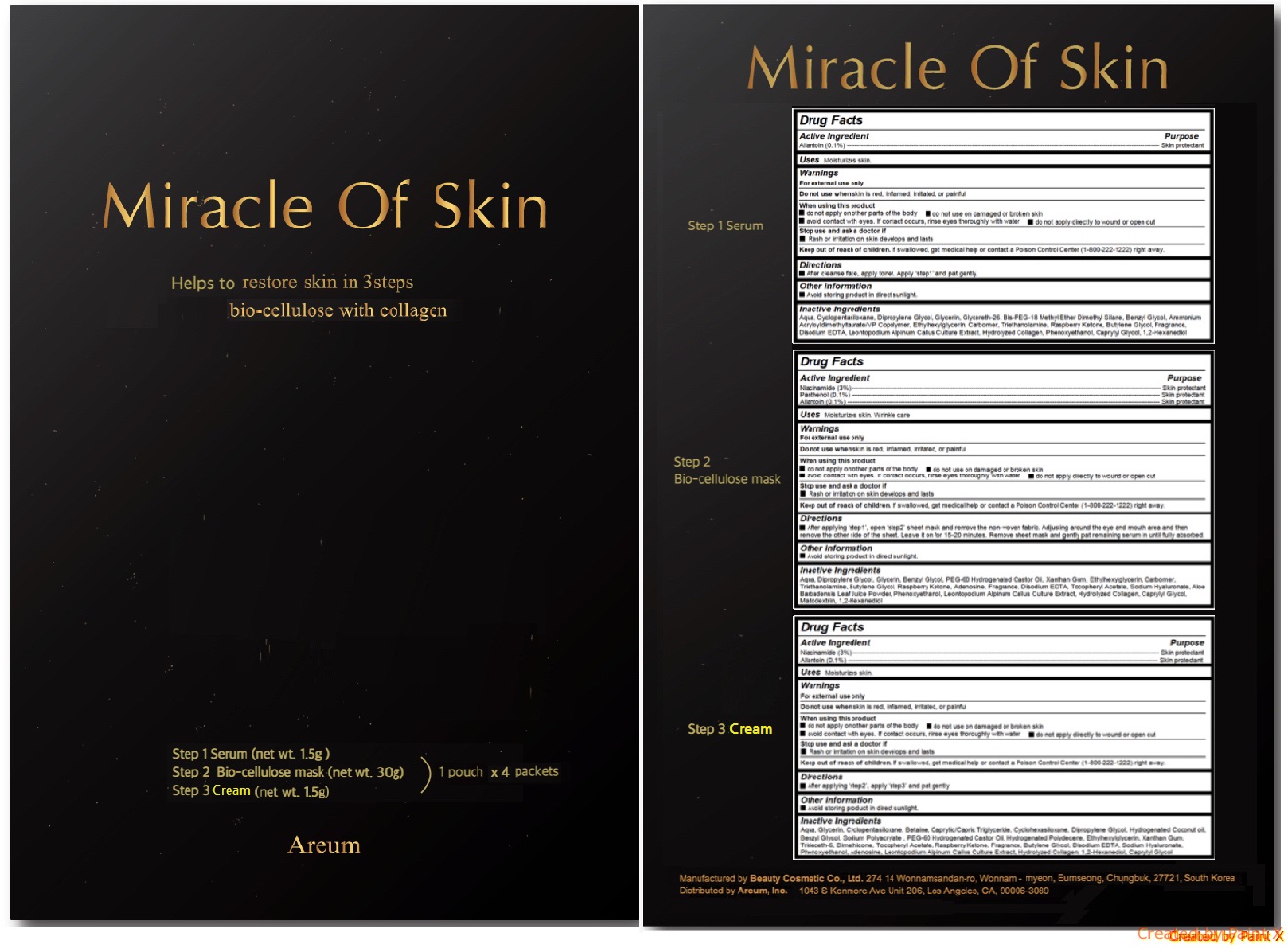
- Active ingredients
- Purpose
- Keep out of reach of children
- Uses
-
Warnings
For external use only
Do not use when skin is red, inflamed, irritated, or painful
When using this product- do not apply on other parts of the body
- do not use on damaged or broken skin
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water
- do not apply directly to wound or open cut
Stop use and ask a doctor if
- Rash or irritation on skin develops and lasts
-
Directions
Step 1 Serum - After cleanse face, apply toner. Apply ‘step1’ and pat gently.
Step 2 Bio-cellulose mask - After applying ‘step1’, open ‘step2’ sheet mask and remove the non-woven fabric. Adjusting around the eye and mouth area and then remove the other side of the sheet. Leave it on for 15-20 minutes. Remove sheet mask and gently pat remaining serum in until fully absorbed.
Step 3 Serum - After applying ‘step2’, apply ‘step3’ and pat gently.
-
INACTIVE INGREDIENT
Step 1 Serum - Aqua, Cyclopentasiloxane, Dipropylene Glycol, Glycerin, Glycereth-26, Bis-PEG-18 Methyl Ether Dimethyl Silane, Benzyl Glycol, Ammonium Acryloyldimethyltaurate/VP Copolymer, Ethylhexylglycerin, Carbomer, Triethanolamine, Raspberry Ketone, Butylene Glycol, Fragrance, Disodium EDTA, Leontopodium Alpinum Callus Culture Extract, Hydrolyzed Collagen, Phenoxyethanol, Caprylyl Glycol, 1,2-Hexanediol
Step 2 Bio-cellulose mask - Aqua, Dipropylene Glycol, Glycerin, Benzyl Glycol, PEG-60 Hydrogenated Castor Oil, Xanthan Gum, Ethylhexylglycerin, Carbomer, Triethanolamine, Butylene Glycol, Raspberry Ketone, Adenosine, Fragrance, Disodium EDTA, Tocopheryl Acetate, Sodium Hyaluronate, Aloe Barbadensis Leaf Juice Powder, Phenoxyethanol, Leontopodium Alpinum Callus Culture Extract, Hydrolyzed Collagen, Caprylyl Glycol, Maltodextrin, 1,2-Hexanediol
Step 3 Serum - Aqua, Glycerin, Cyclopentasiloxane, Betaine, Caprylic/Capric Triglyceride, Cyclohexasiloxane, Dipropylene Glycol, Hydrogenated Coconut oil, Benzyl Glycol, Sodium Polyacrylate , PEG-60 Hydrogenated Castor Oil, Hydrogenated Polydecene, Ethylhexylglycerin, Xanthan Gum, Trideceth-6, Dimethicone, Tocopheryl Acetate, Raspberry Ketone, Fragrance, Butylene Glycol, Disodium EDTA, Sodium Hyaluronate, Phenoxyethanol, Adenosine, Leontopodium Alpinum Callus Culture Extract, Hydrolyzed Collagen, 1,2-Hexanediol, Caprylyl Glycol
- Miracle Of Skin
-
INGREDIENTS AND APPEARANCE
MIRACLE OF SKIN
allantoin, niacinamide, pantheon kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72148-103 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72148-103-01 1 in 1 PACKAGE; Type 1: Convenience Kit of Co-Package 03/15/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 1.5 g Part 2 1 PATCH 30 g Part 3 1 BOTTLE 1.5 g Part 1 of 3 STEP 1 SERUM
allantoin liquidProduct Information Item Code (Source) NDC:72148-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength allantoin (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) allantoin 0.0015 g in 1.5 g Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Dipropylene Glycol (UNII: E107L85C40) Glycerin (UNII: PDC6A3C0OX) BIS-PEG-18 METHYL ETHER DIMETHYL SILANE (UNII: OEB4R3WW9C) ETHYLENE GLYCOL MONOBENZYL ETHER (UNII: 06S8147L47) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) TROLAMINE (UNII: 9O3K93S3TK) 4-(P-HYDROXYPHENYL)-2-BUTANONE (UNII: 7QY1MH15BG) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) 1,2-Hexanediol (UNII: TR046Y3K1G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72148-100-03 4 in 1 PACKAGE 1 NDC:72148-100-02 1 in 1 POUCH 1 NDC:72148-100-01 1.5 g in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/15/2018 Part 2 of 3 STEP 2 BIO-CELLULOSE MASK
niacinamide, panthenol, allantoin patchProduct Information Item Code (Source) NDC:72148-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 0.9 g in 30 g PANTHENOL (UNII: WV9CM0O67Z) (PANTHENOL - UNII:WV9CM0O67Z) PANTHENOL 0.03 g in 30 g Allantoin (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) Allantoin 0.03 g in 30 g Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Dipropylene Glycol (UNII: E107L85C40) Glycerin (UNII: PDC6A3C0OX) ETHYLENE GLYCOL MONOBENZYL ETHER (UNII: 06S8147L47) PEG-60 Hydrogenated Castor Oil (UNII: 02NG325BQG) Xanthan Gum (UNII: TTV12P4NEE) Ethylhexylglycerin (UNII: 147D247K3P) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) TROLAMINE (UNII: 9O3K93S3TK) Butylene Glycol (UNII: 3XUS85K0RA) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALOE VERA LEAF (UNII: ZY81Z83H0X) Phenoxyethanol (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Maltodextrin (UNII: 7CVR7L4A2D) 1,2-Hexanediol (UNII: TR046Y3K1G) 4-(P-HYDROXYPHENYL)-2-BUTANONE (UNII: 7QY1MH15BG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72148-101-02 1 in 1 POUCH 1 NDC:72148-101-01 30 g in 1 PATCH; Type 1: Convenience Kit of Co-Package 2 NDC:72148-101-03 4 in 1 PACKAGE 2 NDC:72148-101-01 30 g in 1 PATCH; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/15/2018 Part 3 of 3 STEP 3
niacinamide, allantoin creamProduct Information Item Code (Source) NDC:72148-102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 0.045 g in 1.5 g allantoin (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) allantoin 0.0015 g in 1.5 g Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) Betaine (UNII: 3SCV180C9W) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 6 (UNII: XHK3U310BA) Dipropylene Glycol (UNII: E107L85C40) Hydrogenated Coconut oil (UNII: JY81OXM1OM) ETHYLENE GLYCOL MONOBENZYL ETHER (UNII: 06S8147L47) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) PEG-60 Hydrogenated Castor Oil (UNII: 02NG325BQG) HYDROGENATED POLYDECENE (550 MW) (UNII: U333RI6EB7) Ethylhexylglycerin (UNII: 147D247K3P) Xanthan Gum (UNII: TTV12P4NEE) Trideceth-6 (UNII: 3T5PCR2H0C) Dimethicone (UNII: 92RU3N3Y1O) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 4-(P-HYDROXYPHENYL)-2-BUTANONE (UNII: 7QY1MH15BG) Butylene Glycol (UNII: 3XUS85K0RA) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Phenoxyethanol (UNII: HIE492ZZ3T) Adenosine (UNII: K72T3FS567) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) Caprylyl Glycol (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72148-102-02 1 in 1 POUCH 1 NDC:72148-102-01 1.5 g in 1 BOTTLE; Type 1: Convenience Kit of Co-Package 2 NDC:72148-102-03 4 in 1 PACKAGE 2 NDC:72148-102-01 1.5 g in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/15/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/15/2018 Labeler - Areum, Inc. (081077378) Registrant - Areum, Inc. (081077378) Establishment Name Address ID/FEI Business Operations Areum, Inc. 081077378 relabel(72148-103) Establishment Name Address ID/FEI Business Operations Beauty Cosmetic Co., Ltd. 688305761 manufacture(72148-103)