TRAMADOL HYDROCHLORIDE EXTENDED RELEASE
- tramadol hydrochloride capsule, extended release
Galephar Pharmaceutical Research Inc.
----------
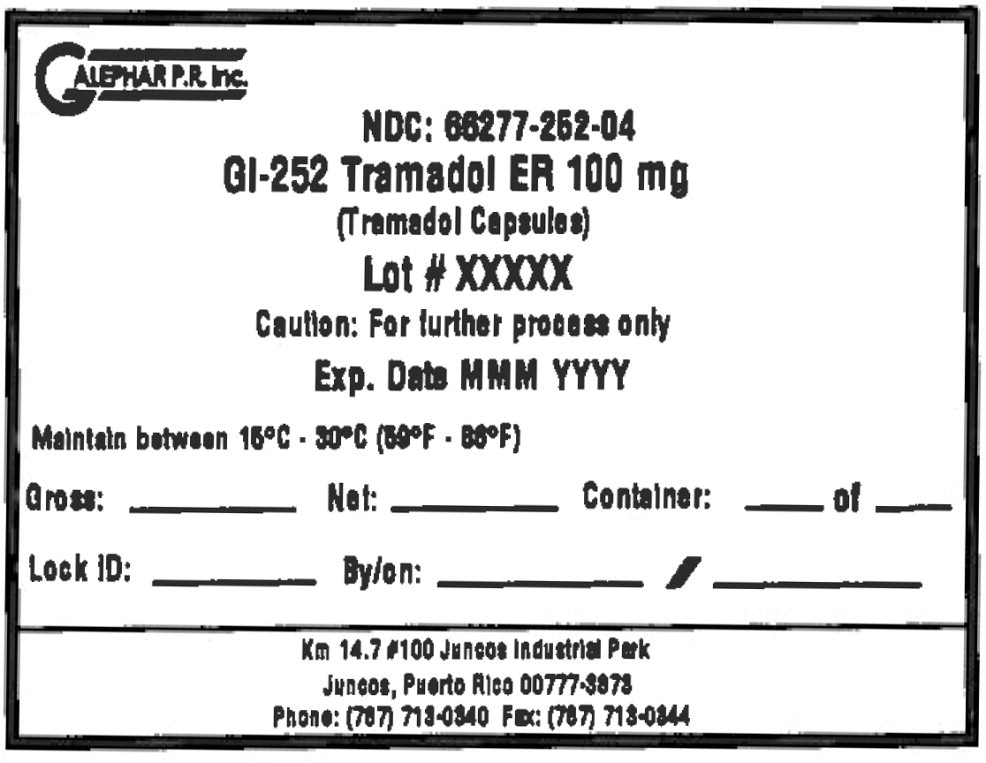
Principal Display Panel - Drum Label
GALEPHAR P.R. Inc.
NDC: 66277-252-04
GI-252 Tramadol ER 100 mg
(Tramadol Capsules)
Lot # XXXXX
Caution: For further process only
Exp. Date MMM YYYY
Maintain between 15°C • 30°C (59°F • 86°F)
Gross: Net: Container: of
Lock ID: By/on: /
Km 14.7 #100 Juncos Industrial Park
Juncos, Puerto Rico 00777-3878
Phone: (787) 713-0340 Fax: (787) 713-0344
Principal Display Panel - Pail label
NDC#: 66277-252-05
Tramadol HCl ER
100 mg CAPSULES
Container of up to 10,000 Capsules
Lot:
Expiration Date:
Gross: kg
Road 198, Km. 14.2 Juncos Industrial Park
Juncos, PR 00777
Phone: (787) 389-0340
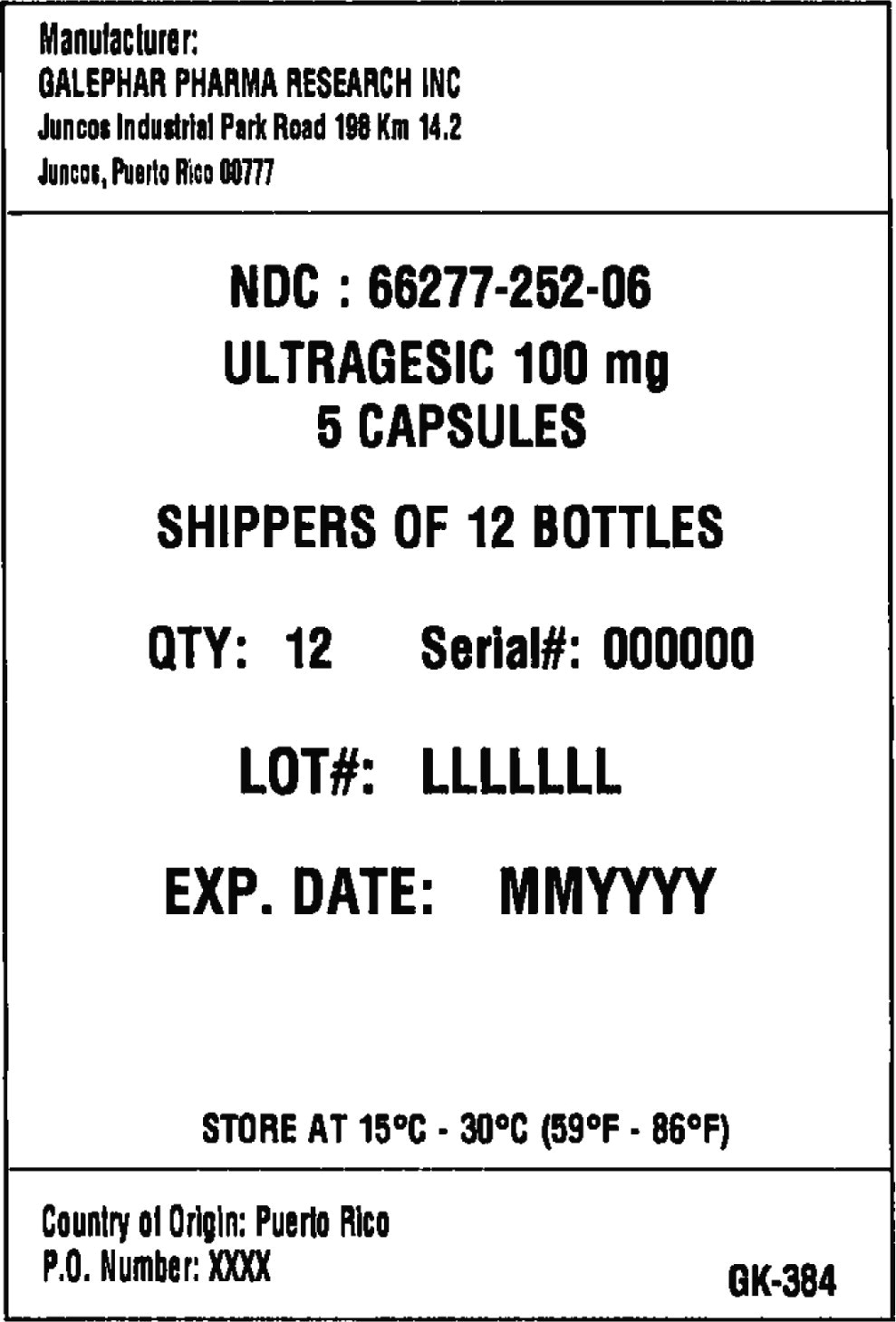
Principal Display Panel - Box Label
Manufacturer:
GALEPHAR PHARMA RESEARCH INC
Juncos Industrial Park Road 198 Km 14.2
Juncos, Puerto Rico 00777
NDC: 66277-252-06
ULTRAGESIC 100 MG
5 CAPSULES
SHIPPERS OF 12 BOTTLES
QTY: 12 Serial#: 000000
LOT#: LLLLLLL
EXP. DATE: MMYYYY
STORE AT 15°C • 30°C (59°F • 86°F)
Country of Origin: Puerto Rico
P.O. Number: XXXX
GK-384
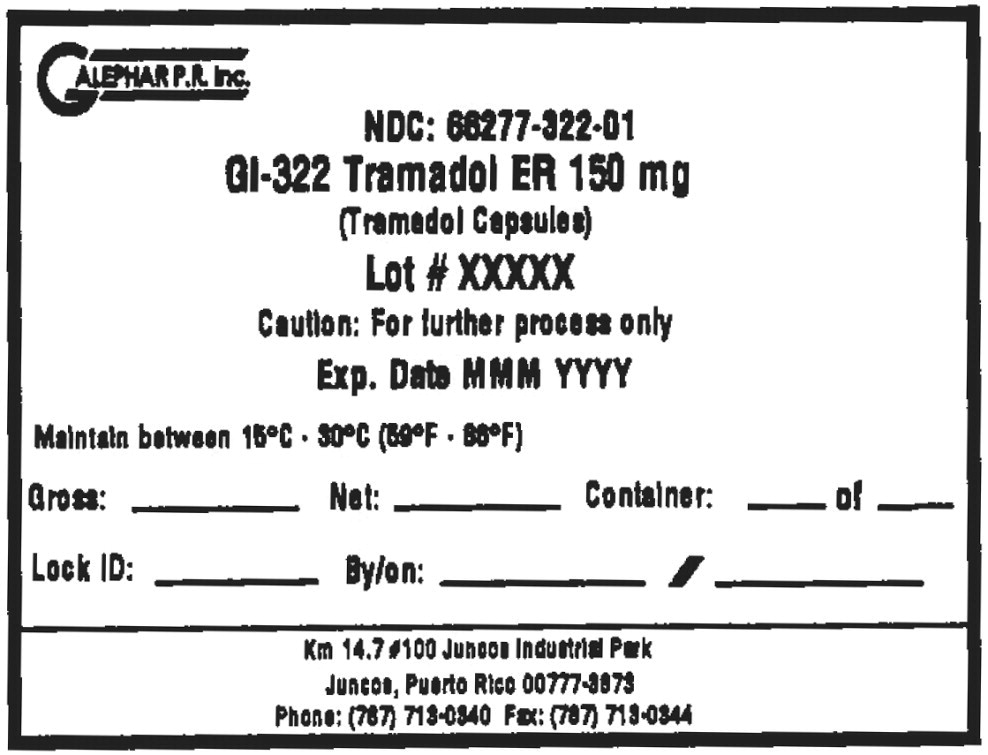
Principal Display Panel - Drum Label
GALEPHAR P.R. Inc.
NDC: 66277-322-01
GI-322 Tramadol ER 150 mg
(Tramadol Capsules)
Lot # XXXXX
Caution: For further process only
Exp. Date MMM YYYY
Maintain between 15°C • 30°C (59°F • 86°F)
Gross: Net: Container: of
Lock ID: By/on: /
km 14.7 #100 Juncos Industrial Park
Juncos, Puerto Rico 00777-3878
Phone: (787) 713-0340 Fax: (787) 713-0344
Principal Display Panel - Pail label
NDC#: 66277-322-02
Tramadol HCl ER
150 mg CAPSULES
Container of up to 10,000 Capsules
Lot:
Expiration Date:
Gross: kg
Road 198, Km. 14.2 Juncos Industrial Park
Juncos, PR 00777
Phone: (787) 389-0340
Principal Display Panel - Box Label
Manufacturer:
GALEPHAR PHARMA RESEARCH INC
Juncos Industrial Park Road 198 Km 14.2
Juncos, Puerto Rico 00777
NDC: 66277-322-04
ULTRAGESIC 150 MG
5 CAPSULES
SHIPPERS OF 12 BOTTLES
QTY: 12 Serial#: 000000
LOT#: LLLLLLL
EXP. DATE: MMYYYY
STORE AT 15°C • 30°C (59°F • 86°F)
Country of Origin: Puerto Rico
P.O. Number: XXXX
GK-385
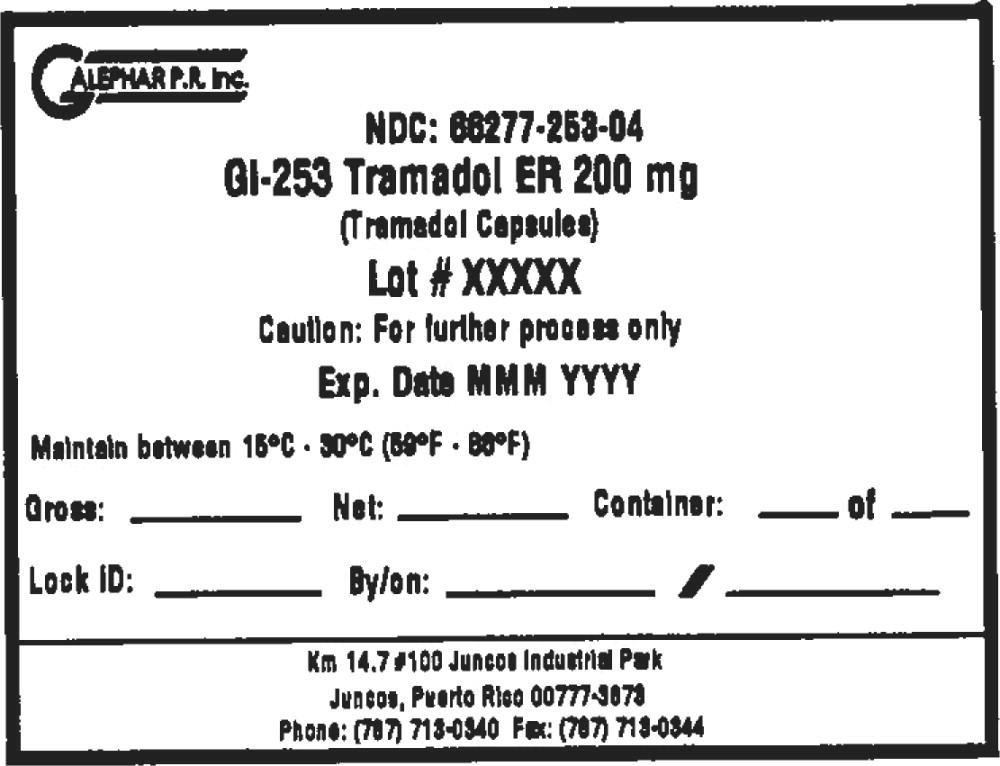
Principal Display Panel - Drum Label
GALEPHAR P.R. Inc.
NDC: 66277-253-04
GI-253 Tramadol ER 200 mg
(Tramadol Capsules)
Lot # XXXXX
Caution: For further process only
Exp. Date MMM YYYY
Maintain between 15°C • 30°C (59°F • 86°F)
Gross: Net: Container: of
Lock ID: By/on: /
km 14.7 #100 Juncos Industrial Park
Juncos, Puerto Rico 00777-3878
Phone: (787) 713-0340 Fax: (787) 713-0344
Principal Display Panel - Pail label
NDC#: 66277-253-05
Tramadol HCl ER
200 mg CAPSULES
Container of up to 10,000 Capsules
Lot:
Expiration Date:
Gross: kg
Road 198, Km. 14.2 Juncos Industrial Park
Juncos, PR 00777
Phone: (787) 389-0340
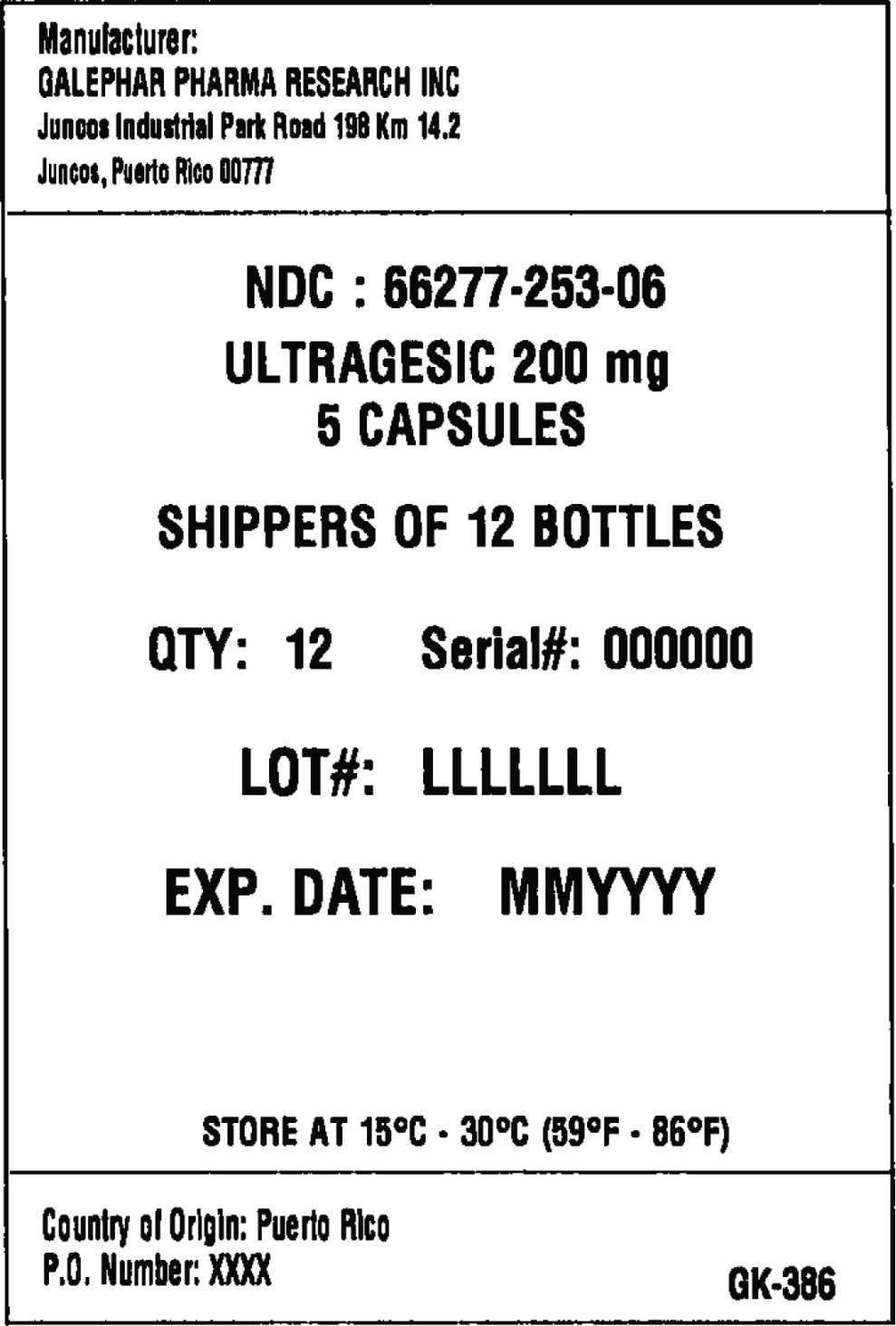
Principal Display Panel - Box Label
Manufacturer:
GALEPHAR PHARMA RESEARCH INC
Juncos Industrial Park Road 198 Km 14.2
Juncos, Puerto Rico 00777
NDC: 66277-253-06
ULTRAGESIC 200 MG
5 CAPSULES
SHIPPERS OF 12 BOTTLES
QTY: 12 Serial#: 000000
LOT#: LLLLLLL
EXP. DATE: MMYYYY
STORE AT 15°C • 30°C (59°F • 86°F)
Country of Origin: Puerto Rico
P.O. Number: XXXX
GK-386
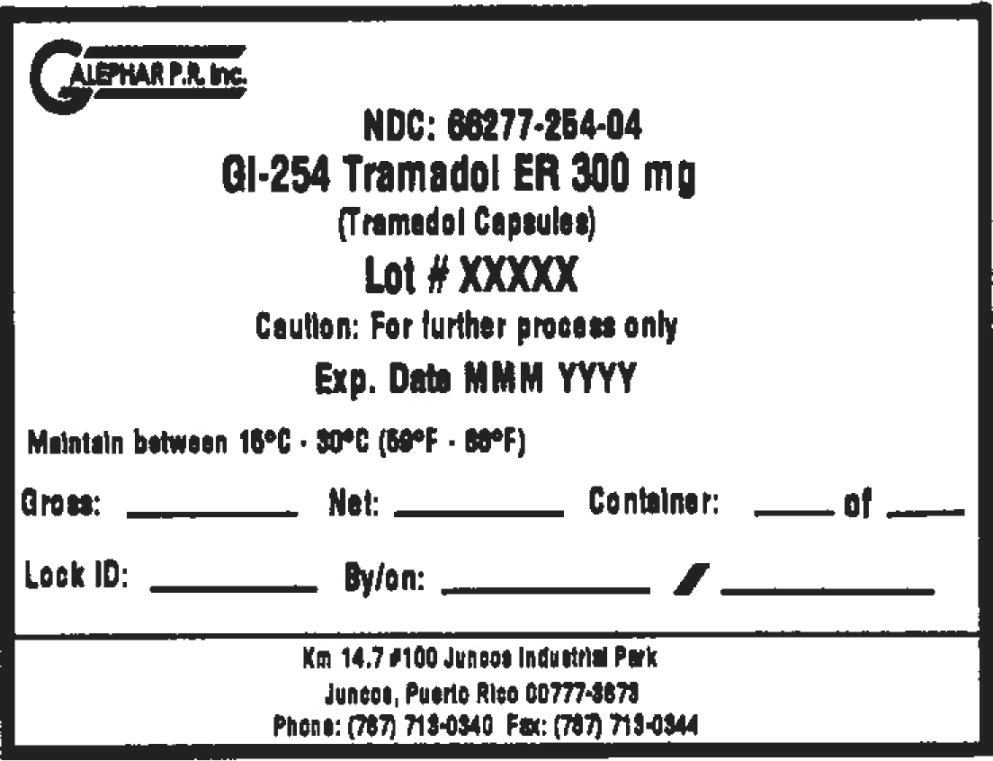
Principal Display Panel - Drum Label
GALEPHAR P.R. Inc.
NDC: 66277-254-04
GI-254 Tramadol ER 300 mg
(Tramadol Capsules)
Lot # XXXXX
Caution: For further process only
Exp. Date MMM YYYY
Maintain between 15°C • 30°C (59°F • 86°F)
Gross: Net: Container: of
Lock ID: By/on: /
km 14.7 #100 Juncos Industrial Park
Juncos, Puerto Rico 00777-3878
Phone: (787) 713-0340 Fax: (787) 713-0344
| TRAMADOL HYDROCHLORIDE
EXTENDED RELEASE
tramadol hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| TRAMADOL HYDROCHLORIDE
EXTENDED RELEASE
tramadol hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| TRAMADOL HYDROCHLORIDE
EXTENDED RELEASE
tramadol hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| TRAMADOL HYDROCHLORIDE
EXTENDED RELEASE
tramadol hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Galephar Pharmaceutical Research Inc. (003551624) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Galephar Pharmaceutical Research Inc. | 003551624 | MANUFACTURE(66277-252, 66277-322, 66277-253, 66277-254) , ANALYSIS(66277-252, 66277-322, 66277-253, 66277-254) , PACK(66277-252, 66277-322, 66277-253, 66277-254) | |