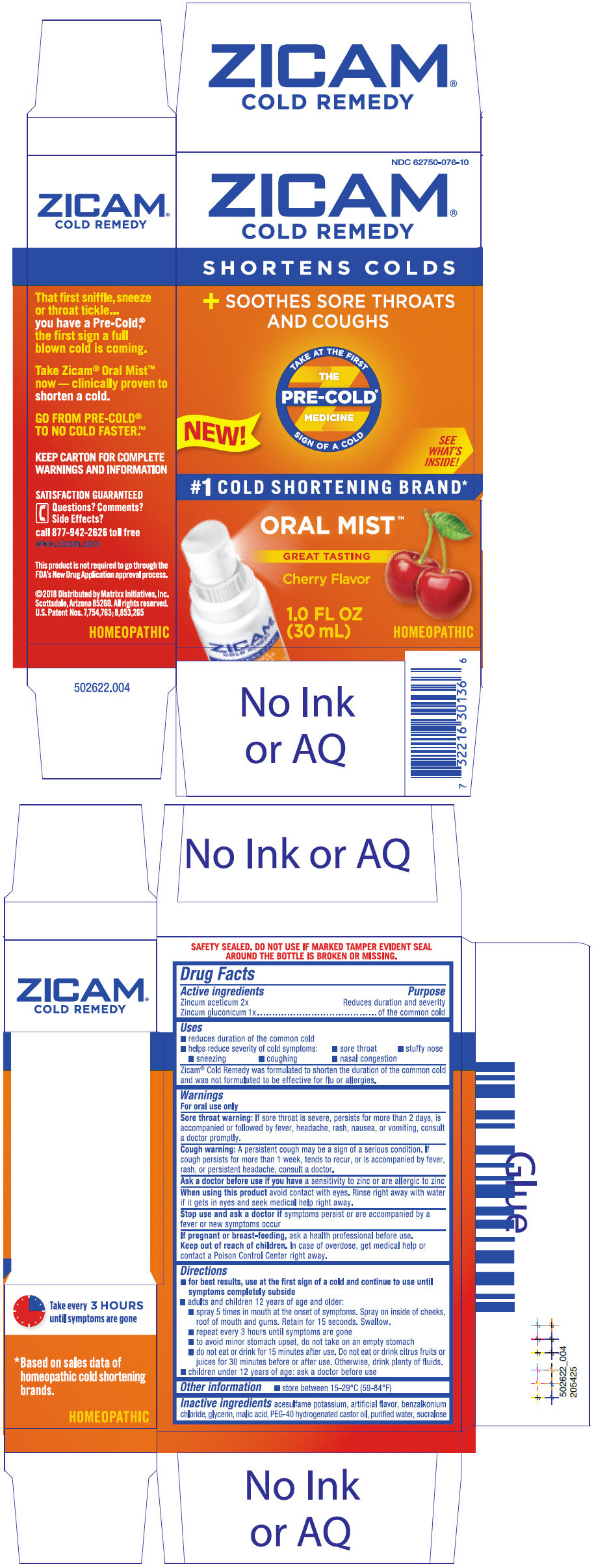
ZICAM COLD REMEDY MIST- zinc acetate anhydrous and zinc gluconate spray
Matrixx Initiatives, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active ingredients
Zincum aceticum 2x
Zincum gluconicum 1x
Purpose
Reduces duration and severity of the common cold
Uses
- reduces duration of the common cold
- helps reduce severity of cold symptoms:
- sore throat
- stuffy nose
- sneezing
- coughing
- nasal congestion
Zicam® Cold Remedy was formulated to shorten the duration of the common cold and was not formulated to be effective for flu or allergies.
Warnings
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Cough warning
A persistent cough may be a sign of a serious condition. If cough persists for more than 1 week, tends to recur, or is accompanied by fever, rash, or persistent headache, consult a doctor.
Ask a doctor before use if you have a sensitivity to zinc or are allergic to zinc
When using this product avoid contact with eyes. Rinse right away with water if it gets in eyes and seek medical help right away.
Stop use and ask a doctor if symptoms persist or are accompanied by a fever or new symptoms occur
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
-
for best results, use at the first sign of a cold and continue to use until symptoms completely subside
- adults and children 12 years of age and older:
- spray 5 times in mouth at the onset of symptoms. Spray on inside of cheeks, roof of mouth and gums. Retain for 15 seconds. Swallow.
- repeat every 3 hours until symptoms are gone
- to avoid minor stomach upset, do not take on an empty stomach
- do not eat or drink for 15 minutes after use. Do not eat or drink citrus fruits or juices for 30 minutes before or after use. Otherwise, drink plenty of fluids.
- children under 12 years of age: ask a doctor before use
Other information
- store between 15-29°C (59-84°F)
Inactive ingredients
acesulfame potassium, artificial flavor, benzalkonium chloride, glycerin, malic acid, PEG-40 hydrogenated castor oil, purified water, sucralose
Questions? Comments? Side Effects?
call 877-942-2626 toll free
www.zicam.com
Distributed by Matrixx Initiatives, Inc.
Scottsdale, Arizona 85260.
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton
NDC 62750-076-10
ZICAM®
COLD REMEDY
SHORTENS COLDS
+ SOOTHES SORE THROATS
AND COUGHS
TAKE AT THE FIRST
THE
PRE-COLD®
MEDICINE
SIGN OF A COLD
NEW!
SEE
WHAT'S
INSIDE!
#1 COLD SHORTENING BRAND*
ORAL MIST™
GREAT TASTING
Cherry Flavor
1.0 FL OZ
(30 mL)
HOMEOPATHIC
Matrixx Initiatives, Inc.