Label: LINCOMIX 50- lincomycin hydrochloride granule
- NDC Code(s): 54771-1494-2
- Packager: Zoetis Inc.
- Category: VFD TYPE A MEDICATED ARTICLE ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated December 8, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
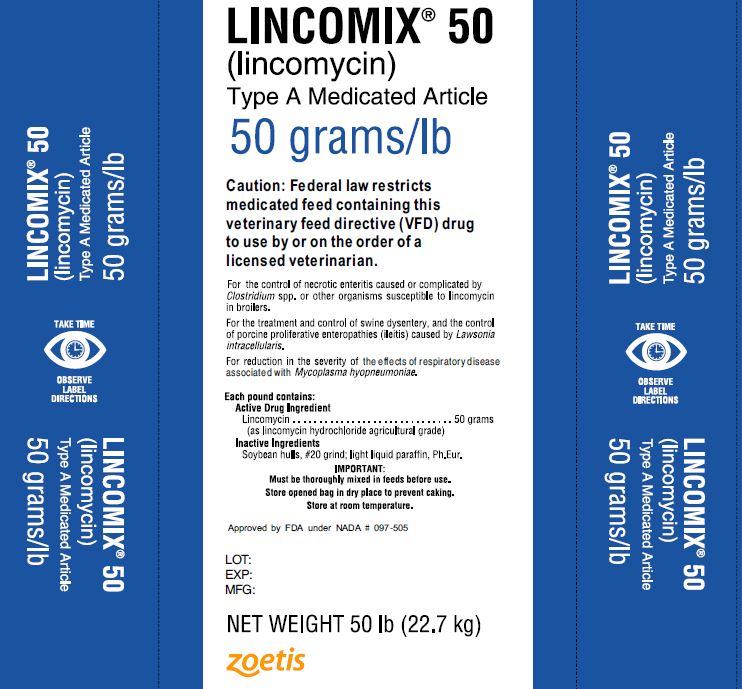
Type A Medicated Article
50 grams/lb
Caution: Federal law restricts medicated feed containing this veterinary feed directive (VFD) drug to use by or on the order of a licensed veterinarian.
For the control of necrotic enteritis caused or complicated by Clostridium spp. or other organisms susceptible to lincomycin in broilers.
For the treatment and control of swine dysentery, and the control of porcine proliferative enteropathies (ileitis) casued by Lawsonia intracellularis.
For reduction in the severity of the effects of respiratory disease associated with Mycoplasma hyopneumoniae.
Each pound contains:
Active Drug Ingredient
Lincomycin………………………………..50 grams
(as lincomycin hydrochloride agricultural grade)
Inactive Ingredients
Soybean hulls, #20 grind; light liquid paraffin, Ph.Eur.
IMPORTANT:
Must be thoroughly mixed in feeds before use.
Store opened bag in dry place to prevent caking.
Store at room temperature.
Approved by FDA under NADA # 097-505
-
BROILERS
BROILERS
For the control of necrotic enteritis caused or complicated by Clostridium spp. or other organisms susceptible to lincomycin in broilers.
DIRECTIONS FOR USE
For the control of necrotic enteritis:
LINCOMIX 50, 50 grams/lb, should be mixed into the complete feed supplied to broiler chickens so that the final feed contains 2 grams of lincomycin per ton of feed.
MIXING DIRECTIONS
Type C Medicated Feeds
Intermediate Premix
Amount of LINCOMIX 50 per 1000 lb
(454 kg) of Feed IngredientsComplete Feed
Amount of Intermediate Premix to Provide 2 Grams
of lincomycin per ton of Type C Medicated Feed
lincomycin per ton of feed
2 grams
20 lbs
4 lbs
2 lbs
2 lbs
10 lbs
20 lbs
WARNING: When using LINCOMIX 50 in approved combinations with other drugs, follow the required withdrawal times for those drugs. No drug withdrawal period is required before slaughter of birds fed LINCOMIX 50 at the approved concentration (2 grams lincomycin per ton of feed).
CAUTION: Not for use in layers, breeders, or turkeys. (For additional Cautions, see below.)
-
SWINE
SWINE
For the treatment and control of swine dysentery, and the control of porcine proliferative enteropathies (ileitis) caused by Lawsonia intracellularis. For reduction in the severity of the effects of respiratory disease associated with Mycoplasma hyopneumoniae.
DIRECTIONS FOR USE
For the treatment of swine dysentery, and the control of porcine proliferative enteropathies (ileitis) caused by Lawsonia intracellularis:
Feed 100 grams of lincomycin per ton of complete feed as the sole ration for three weeks, or until signs of disease (watery, mucoid or bloody stools) disappear.
For the treatment and control of swine dysentery, and the control of porcine proliferative enteropathies (ileitis) caused by Lawsonia intracellularis:
Feed 100 grams of lincomycin per ton of complete feed as the sole ration for three weeks, or until signs of disease (watery, mucoid or bloody stools) disappear, followed by 40 grams of lincomycin per ton.
For the control of swine dysentery and porcine proliferative enteropathies (ileitis) caused by Lawsonia intracellularis: Feed 40 grams of lincomycin per ton of complete feed as the sole ration. For use in animals or on premises with a history of swine dysentery, but where symptoms have not yet occurred.
For reduction in the severity of the effects of respiratory disease associated with Mycoplasma hyopneumoniae:
Feed 100-200 grams of lincomycin per ton of complete feed as the sole ration for 21 days.
MIXING DIRECTIONS
Type C Medicated Feeds
For treatment of swine dysentery, and the control of porcine proliferative enteropathies (ileitis) caused by Lawsonia intracellularis:
To make complete feed containing 100 grams of lincomycin, add 2 lbs of LINCOMIX 50 per ton.
For control of swine dysentery and porcine proliferative enteropathies (ileitis) caused by Lawsonia intracellularis:
To make complete feed containing 40 grams of lincomycin, add 0.8 lbs of LINCOMIX 50 per ton.
For reduction in the severity of the effects of respiratory disease associated with Mycoplasma hyopneumoniae:
To make complete feed containing 100- 200 grams of lincomycin, add 2-4 lbs of LINCOMIX 50 per ton.
Additional mixing directions to make complete feed containing 40, 100 or 200 grams of lincomycin per ton are provided below.
MIXING DIRECTIONS
IntermediatePremix
Amount of LINCOMIX 50 per 1000 lb
(454 kg) of Feed Ingredients
Complete Feed
Amount of Intermediate Premix to Provide Desired Grams of lincomycin per ton of Type C Medicated Feed
lincomycin per ton of feed
50 lbs
40 lbs
20 lbs
40 grams 100 grams 200 grams
16 lbs 40 lbs 80 lbs
20 lbs 50 lbs 100 lbs
40 lbs 100 lbs 200 lbs
WARNING: When using LINCOMIX 50 in approved combinations with other drugs, follow the required withdrawal times for those drugs. No drug withdrawal period is required before slaughter of swine fed LINCOMIX 50 at approved concentrations (40, 100 or 100-200 grams lincomycin per ton of feed).
NOT FOR HUMAN USE
CAUTION: Occasionally, swine fed lincomycin may within the first two days after the onset of treatment develop diarrhea and/or swelling of the anus. On rare occasions, some pigs may show reddening of the skin and irritable behavior. These conditions have been self-correcting within five to eight days without discontinuing the lincomycin treatment. The effects of lincomycin on swine reproductive performance, pregnancy, and lactation have not been determined.
-
SPL UNCLASSIFIED SECTION
Do not allow rabbits, hamsters, guinea pigs, horses, or ruminants access to feeds containing lincomycin. Ingestion by these species may result in severe gastrointestinal effects.
Good Manufacturing Practices Should Be Observed in Preparing Feeds Containing LINCOMIX 50.
This Includes Appropriate Clean-out Procedures to Avoid Cross-Contamination.
Restricted Drug (California) – Use Only as Directed
Distributed by:
Zoetis Inc.
Kalamazoo, MI 49007501918
40027871
Made in China
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LINCOMIX 50
lincomycin hydrochloride granuleProduct Information Product Type VFD TYPE A MEDICATED ARTICLE ANIMAL DRUG Item Code (Source) NDC:54771-1494 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LINCOMYCIN HYDROCHLORIDE (UNII: M6T05Z2B68) (LINCOMYCIN - UNII:BOD072YW0F) LINCOMYCIN 50 g in 0.453 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-1494-2 22.7 kg in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA097505 06/30/1976 Labeler - Zoetis Inc. (828851555)