Label: BESIVANCE- besifloxacin suspension
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-6282-0 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 24208-446
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 1, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Besivance safely and effectively. See full prescribing information for Besivance.
BesivanceTM (besifloxacin ophthalmic suspension) 0.6%
Sterile topical ophthalmic drops
Initial U.S. Approval: 2009INDICATIONS AND USAGE
Besivance™ (besifloxacin ophthalmic suspension) 0.6%, is a quinolone antimicrobial indicated for the treatment of bacterial conjunctivitis caused by susceptible isolates of the following bacteria:
CDC coryneform group G
Corynebacterium pseudodiphtheriticum*, Corynebacterium striatum*, Haemophilus influenzae, Moraxella lacunata*, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus hominis*, Staphylococcus lugdunensis*, Streptococcus mitis group, Streptococcus oralis, Streptococcus pneumoniae, Streptococcus salivarius*
*Efficacy for this organism was studied in fewer than 10 infections. (1)
DOSAGE AND ADMINISTRATION
Instill one drop in the affected eye(s) 3 times a day, four to twelve hours apart for 7 days. (2)
DOSAGE FORMS AND STRENGTHS
7.5 mL size bottle filled with 5 mL of besifloxacin ophthalmic suspension, 0.6% (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse event reported in 2% of patients treated with Besivance™ was conjunctival redness. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb Incorporated at 1-800-323-0000 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2011
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Topical Ophthalmic Use Only
5.2 Growth of Resistant Organisms with Prolonged Use
5.3 Avoidance of Contact Lenses
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Besivance™ (besifloxacin ophthalmic suspension) 0.6%, is indicated for the treatment of bacterial conjunctivitis caused by susceptible isolates of the following bacteria:
CDC coryneform group G
Corynebacterium pseudodiphtheriticum*
Corynebacterium striatum*
Haemophilus influenzae
Moraxella lacunata*
Staphylococcus aureus
Staphylococcus epidermidis
Staphylococcus hominis*
Staphylococcus lugdunensis*
Streptococcus mitis group
Streptococcus oralis
Streptococcus pneumoniae
Streptococcus salivarius*
*Efficacy for this organism was studied in fewer than 10 infections.
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Topical Ophthalmic Use Only
NOT FOR INJECTION INTO THE EYE.
Besivance™ is for topical ophthalmic use only, and should not be injected subconjunctivally, nor should it be introduced directly into the anterior chamber of the eye.
5.2 Growth of Resistant Organisms with Prolonged Use
As with other anti-infectives, prolonged use of Besivance™ (besifloxacin ophthalmic suspension) 0.6% may result in overgrowth of non-susceptible organisms, including fungi. If super-infection occurs, discontinue use and institute alternative therapy. Whenever clinical judgment dictates, the patient should be examined with the aid of magnification, such as slit-lamp biomicroscopy, and, where appropriate, fluorescein staining.
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in one clinical trial of a drug cannot be directly compared with the rates in the clinical trials of the same or another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to Besivance™ in approximately 1,000 patients between 1 and 98 years old with clinical signs and symptoms of bacterial conjunctivitis.
The most frequently reported ocular adverse event was conjunctival redness, reported in approximately 2% of patients.
Other adverse events reported in patients receiving Besivance™ occurring in approximately 1-2% of patients included: blurred vision, eye pain, eye irritation, eye pruritus and headache.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. Oral doses of besifloxacin up to 1000 mg/kg/day were not associated with visceral or skeletal malformations in rat pups in a study of embryo-fetal development, although this dose was associated with maternal toxicity (reduced body weight gain and food consumption) and maternal mortality. Increased post-implantation loss, decreased fetal body weights, and decreased fetal ossification were also observed. At this dose, the mean Cmax in the rat dams was approximately 20 mcg/mL, >45,000 times the mean plasma concentrations measured in humans. The No Observed Adverse Effect Level (NOAEL) for this embryo-fetal development study was 100 mg/kg/day (Cmax, 5 mcg/mL, >11,000 times the mean plasma concentrations measured in humans).
In a prenatal and postnatal development study in rats, the NOAELs for both fetal and maternal toxicity were also 100 mg/kg/day. At 1000 mg/kg/day, the pups weighed significantly less than controls and had a reduced neonatal survival rate. Attainment of developmental landmarks and sexual maturation were delayed, although surviving pups from this dose group that were reared to maturity did not demonstrate deficits in behavior, including activity, learning and memory, and their reproductive capacity appeared normal.
Since there are no adequate and well-controlled studies in pregnant women, Besivance™ should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
Besifloxacin has not been measured in human milk, although it can be presumed to be excreted in human milk. Caution should be exercised when Besivance™ is administered to a nursing mother.
8.4 Pediatric Use
The safety and effectiveness of Besivance™ in infants below one year of age have not been established. The efficacy of Besivance™ in treating bacterial conjunctivitis in pediatric patients one year or older has been demonstrated in controlled clinical trials [see 14 CLINICAL STUDIES].
There is no evidence that the ophthalmic administration of quinolones has any effect on weight bearing joints, even though systemic administration of some quinolones has been shown to cause arthropathy in immature animals.
-
11 DESCRIPTION
Besivance™ (besifloxacin ophthalmic suspension) 0.6%, is a sterile ophthalmic suspension of besifloxacin formulated with DuraSite®* (polycarbophil, edetate disodium dihydrate and sodium chloride). Each mL of Besivance™ contains 6.63 mg besifloxacin hydrochloride equivalent to 6 mg besifloxacin base. It is an 8-chloro fluoroquinolone anti-infective for topical ophthalmic use.
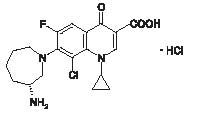
C19H21ClFN3O3•HCl
Mol Wt 430.30
Chemical Name: (+)-7-[(3R)-3-aminohexahydro-1H-azepin-1-yl]-8-chloro-1- cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid hydrochloride.
Besifloxacin hydrochloride is a white to pale yellowish-white powder.
Each mL Contains:
Active: besifloxacin 0.6% (6 mg/mL);
Preservative: benzalkonium chloride 0.01%
Inactives: polycarbophil, mannitol, poloxamer 407, sodium chloride, edetate disodium dihydrate, sodium hydroxide and water for injection.
Besivance™ is an isotonic suspension with an osmolality of approximately 290 mOsm/kg.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Besifloxacin is a fluoroquinolone antibacterial [see 12.4 Clinical Pharmacology, Microbiology].
12.3 Pharmacokinetics
Plasma concentrations of besifloxacin were measured in adult patients with suspected bacterial conjunctivitis who received Besivance™ bilaterally three times a day (16 doses total). Following the first and last dose, the maximum plasma besifloxacin concentration in each patient was less than 1.3 ng/mL. The mean besifloxacin Cmax was 0.37 ng/mL on day 1 and 0.43 ng/mL on day 6. The average elimination half-life of besifloxacin in plasma following multiple dosing was estimated to be 7 hours.
12.4 Microbiology
Besifloxacin is an 8-chloro fluoroquinolone with a N-1 cyclopropyl group. The compound has activity against Gram-positive and Gram-negative bacteria due to the inhibition of both bacterial DNA gyrase and topoisomerase IV. DNA gyrase is an essential enzyme required for replication, transcription and repair of bacterial DNA. Topoisomerase IV is an essential enzyme required for partitioning of the chromosomal DNA during bacterial cell division. Besifloxacin is bactericidal with minimum bactericidal concentrations (MBCs) generally within one dilution of the minimum inhibitory concentrations (MICs).
The mechanism of action of fluoroquinolones, including besifloxacin, is different from that of aminoglycoside, macrolide, and β-lactam antibiotics. Therefore, besifloxacin may be active against pathogens that are resistant to these antibiotics and these antibiotics may be active against pathogens that are resistant to besifloxacin. In vitro studies demonstrated crossresistance between besifloxacin and some fluoroquinolones.
In vitro resistance to besifloxacin develops via multiple-step mutations and occurs at a general frequency of < 3.3 x 10-10 for Staphylococcus aureus and < 7 x 10-10 for Streptococcus pneumoniae.
Besifloxacin has been shown to be active against most isolates of the following bacteria both in vitro and in conjunctival infections treated in clinical trials as described in the INDICATIONS AND USAGE section:
CDC coryneform group G
Corynebacterium pseudodiphtheriticum*
Corynebacterium striatum*
Haemophilus influenzae
Moraxella lacunata*
Staphylococcus aureus
Staphylococcus epidermidis
Staphylococcus hominis*
Staphylococcus lugdunensis*
Streptococcus mitis group
Streptococcus oralis
Streptococcus pneumoniae
Streptococcus salivarius*
*Efficacy for this organism was studied in fewer than 10 infections.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to determine the carcinogenic potential of besifloxacin have not been performed.
No in vitro mutagenic activity of besifloxacin was observed in an Ames test (up to 3.33 mcg/plate) on bacterial tester strains Salmonella typhimurium TA98, TA100, TA1535, TA1537 and Escherichia coli WP2uvrA. However, it was mutagenic in S. typhimurium strain TA102 and E. coli strain WP2(pKM101). Positive responses in these strains have been observed with other quinolones and are likely related to topoisomerase inhibition.
Besifloxacin induced chromosomal aberrations in CHO cells in vitro and it was positive in an in vivo mouse micronucleus assay at oral doses ≥ 1500 mg/kg. Besifloxacin did not induce unscheduled DNA synthesis in hepatocytes cultured from rats given the test compound up to 2,000 mg/ kg by the oral route. In a fertility and early embryonic development study in rats, besifloxacin did not impair the fertility of male or female rats at oral doses of up to 500 mg/kg/day. This is over 10,000 times higher than the recommended total daily human ophthalmic dose.
-
14 CLINICAL STUDIES
In a randomized, double-masked, vehicle controlled, multicenter clinical trial, in which patients 1-98 years of age were dosed 3 times a day for 5 days, Besivance™ was superior to its vehicle in patients with bacterial conjunctivitis. Clinical resolution was achieved in 45% (90/198) for the Besivance™ treated group versus 33% (63/191) for the vehicle treated group (difference 12%, 95% CI 3% - 22%). Microbiological outcomes demonstrated a statistically significant eradication rate for causative pathogens of 91% (181/198) for the Besivance™ treated group versus 60% (114/191) for the vehicle treated group (difference 31%, 95% CI 23% - 40%). Microbiologic eradication does not always correlate with clinical outcome in anti-infective trials.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Besivance™ (besifloxacin ophthalmic suspension) 0.6%, is supplied as a sterile ophthalmic suspension in a white low density polyethylene (LDPE) bottle with a controlled dropper tip and tan polypropylene cap. Tamper evidence is provided with a shrink band around the cap and neck area of the package.
5 mL in 7.5 mL bottle
NDC 54868-6282-0
Storage: Store at 15°- 25°C (59° - 77°F). Protect from Light.Invert closed bottle and shake once before use.
Rx Only
-
17 PATIENT COUNSELING INFORMATION
Patients should be advised to avoid contaminating the applicator tip with material from the eye, fingers or other source.
Although Besivance™ is not intended to be administered systemically, quinolones administered systemically have been associated with hypersensitivity reactions, even following a single dose. Patients should be advised to discontinue use immediately and contact their physician at the first sign of a rash or allergic reaction.
Patients should be told that although it is common to feel better early in the course of the therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Besivance™ or other antibacterial drugs in the future.
Patients should be advised not to wear contact lenses if they have signs or symptoms of bacterial conjunctivitis or during the course of therapy with Besivance™.
Patients should be advised to thoroughly wash hands prior to using Besivance™.
Patients should be instructed to invert closed bottle (upside down) and shake once before each use. Remove cap with bottle still in the inverted position. Tilt head back, and with bottle inverted, gently squeeze bottle to instill one drop into the affected eye(s).
MANUFACTURER INFORMATIONManufactured by: Bausch & Lomb Incorporated
Tampa, Florida 33637
©Bausch & Lomb IncorporatedU.S. Patent No. 6,685,958
U.S. Patent No. 6,699,492
U.S. Patent No. 5,447,926Besivance™ is a trademark of Bausch & Lomb Incorporated
*DuraSite is a trademark of InSite Vision Incorporated
April 2009
Relabeling and Repackaging by:
Physicians Total Care, Inc.
Tulsa, OK 74146
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BESIVANCE
besifloxacin suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-6282(NDC:24208-446) Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BESIFLOXACIN (UNII: BFE2NBZ7NX) (BESIFLOXACIN - UNII:BFE2NBZ7NX) BESIFLOXACIN 6 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) POLYCARBOPHIL (UNII: W25LM17A4W) MANNITOL (UNII: 3OWL53L36A) POLOXAMER 407 (UNII: TUF2IVW3M2) SODIUM CHLORIDE (UNII: 451W47IQ8X) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-6282-0 1 in 1 CARTON 1 5 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022308 07/13/2011 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel, repack


