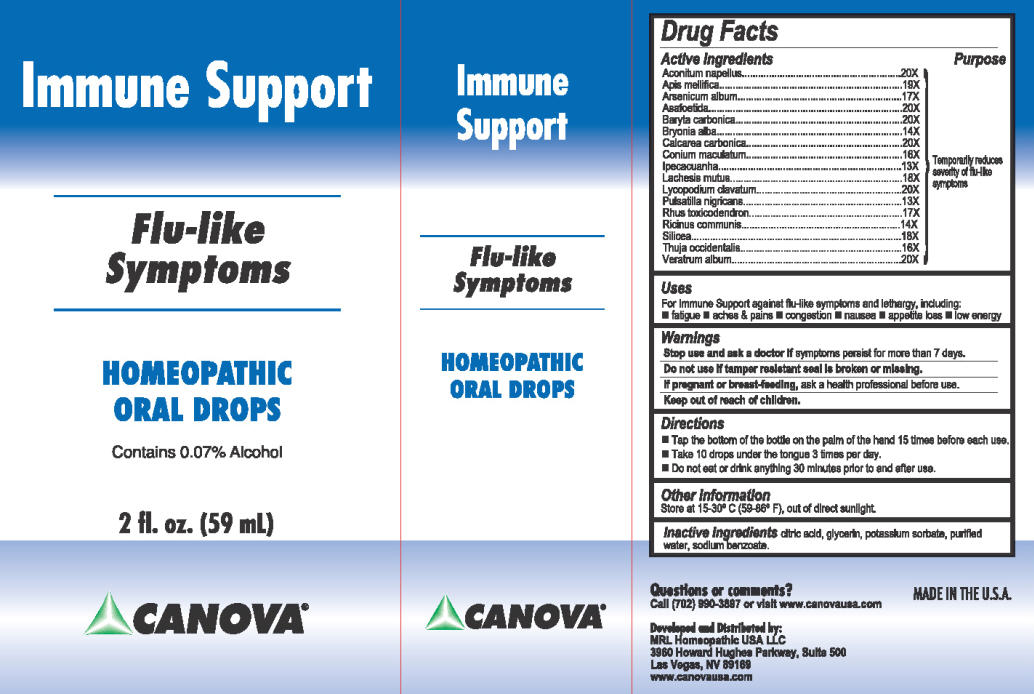
Label: CANOVA IMMUNE SUPPORT liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 58088-001-01 - Packager: MRL Homeopathic USA LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated May 8, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient
Aconitum napellus..............20X
Apis mellifica......................19X
Arsenicum album................17X
Asafoetida...........................20X
Baryta carbonica.................20X
Bryonia alba........................14X
Calcarea carbonica..............20X
Conium maculatum.............16X
Ipecacuanha.........................13X
Lachesis mutus....................18X
Lycopodium clavatum.........20X
Pulsatilla nigricans...............13X
Rhus toxicodendron.............17X
Ricinus communis...............14X
Silicea..................................18X
Thuja occidentalis................16X
Veratrum album...................20X
- Purpose
- Uses
- Warnings
- Keep out of reach of children
- Directions
- Other Information
- Inactive ingredients
- Questions or comments?
-
Patient Information
When was Canova Immune Support developed?
In the 1960s, Dr. Francisco Canova, an Argentinean medical doctor and trained homeopath, developed a homeopathic medicine, later called Canova Immune Support, for the debilitating effects of a compromised immune system. Canova Immune Support developed a loyal following reaching over 10,000 users by the early 1990s.
What is Canova Immune Support?
Canova Immune Support is a homeopathic medicine made from 17 different plants, mineral and animal ingredients. It provides favorable support to your immune system in your day-to-day activities by boosting the body's immune system and enhancing resistance to immune challenges due to fatigue, exposure to environmental challenges, and stress. Canova Immune Support is a complement, not replacement, to traditional (allopathic) medicine.
Can Canova Immune Support be used to treat recurring illnesses?
For patients with recurring illnesses, Canova Immune Support aims to reduce the intensity and frequency of episodes or outbreaks, as with allergies or herpes, for example.*
What is the main benefit of taking Canova Immune Support?
Canova Immune Support stimulates the human body’s defenses against infection, disease, or other unwanted biological invasion, providing relief for symptoms experienced by people with a weakened immune system, such as fatigue, nausea, appetite loss, and aches and pains. The ultimate and main benefit of Canova Immune Support is the improved quality of life for people fighting incapacitating symptoms, such as the severity of those associated with the flu.*
Can Canova Immune Support be taken in conjunction with other medications?
The extremely small doses of each active homeopathic ingredient of Canova Immune Support assure it is safe and has no known side effects or contraindications. Canova Immune Support can be safely taken with other conventional medicines and is usually recommended as a complement to other treatments.*
Can I give Canova Immune Support to my children?
Homeopathic medicines are generally safe for children. Please see package labeling for specific drug facts and dosage recommendations. For a child under the age specified on the label, please check with a doctor or pharmacist first.
Can I use Canova Immune Support by myself, without a doctor's supervision?
Canova Immune Support may be used for self-diagnosable conditions that do not require a doctor’s supervision. Canova Immune Support is clearly labeled with warnings and directions for use, as per FDA Regulations. Always read and follow label directions when taking Canova without a doctor’s supervision.
Is Canova Immune Support safe to use when pregnant?
As with any medicine, pregnant women should seek the advice of a medical professional before use.
How popular is Canova Immune Support?
Canova Immune Support is currently used by an estimated 30,000 people worldwide.
Has Canova Immune Support been researched?
Several Universities and Research Institutions conducted various research studies on Canova Immune Support and published their results in 16 international life science journals, seven university published papers, and 19 international congress papers.
What is the best way to store Canova Immune Support?
The best way to store Canova Immune Support is away from direct sunlight, TV sets, radios, microwave ovens, cell phones, other electrical appliances and fragrant substances, such as perfumes. It is also important that the bottle be tightly closed after each use.
*This statement has not been approved by the FDA. This product is not intended to treat, cure or prevent any disease.
Questions or comments?
Call (702) 990-3897 or visit www.canovausa.comDeveloped and Distributed by:
MRL Homeopathic USA LLC
3960 Howard Hughes Parkway, Suite 500
Las Vegas, NV 89169 www.canovausa.comMADE IN THE U.S.A.
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CANOVA IMMUNE SUPPORT
canova immune support liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58088-001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 20 [hp_X] in 59 mL APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 19 [hp_X] in 59 mL ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC CATION (3+) 17 [hp_X] in 59 mL ASAFETIDA (UNII: W9FZA51AS1) (ASAFETIDA - UNII:W9FZA51AS1) ASAFETIDA 20 [hp_X] in 59 mL BARIUM CARBONATE (UNII: 6P669D8HQ8) (BARIUM CATION - UNII:V645272HLN) BARIUM CARBONATE 20 [hp_X] in 59 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 14 [hp_X] in 59 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 20 [hp_X] in 59 mL CONIUM MACULATUM FLOWERING TOP (UNII: Q28R5GF371) (CONIUM MACULATUM FLOWERING TOP - UNII:Q28R5GF371) CONIUM MACULATUM FLOWERING TOP 16 [hp_X] in 59 mL IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 13 [hp_X] in 59 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 18 [hp_X] in 59 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 20 [hp_X] in 59 mL PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 13 [hp_X] in 59 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 17 [hp_X] in 59 mL CASTOR OIL (UNII: D5340Y2I9G) (CASTOR OIL - UNII:D5340Y2I9G) CASTOR OIL 14 [hp_X] in 59 mL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 18 [hp_X] in 59 mL THUJA OCCIDENTALIS ROOT (UNII: 12958C0BR3) (THUJA OCCIDENTALIS ROOT - UNII:12958C0BR3) THUJA OCCIDENTALIS ROOT 16 [hp_X] in 59 mL VERATRUM ALBUM ROOT (UNII: QNS6W5US1Z) (VERATRUM ALBUM ROOT - UNII:QNS6W5US1Z) VERATRUM ALBUM ROOT 20 [hp_X] in 59 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58088-001-01 1 in 1 CARTON 1 59 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved homeopathic 05/06/2013 Labeler - MRL Homeopathic USA LLC (078783063) Establishment Name Address ID/FEI Business Operations Oasis Health Products, Inc. 964858315 MANUFACTURE(58088-001)