SMART SENSE ALLERGY- diphenhydramine hydrochloride tablet
Kmart Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Kmart Corporation Allergy Tablets Drug Facts
Uses
- •
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- •
- runny nose
- •
- sneezing
- •
- itchy, watery eyes
- •
- itchy nose or throat
- •
- temporarily relieves these symptoms of the common cold:
- •
- runny nose
- •
- sneezing
Warnings
Do not use
- •
- with any other product containing diphenhydramine, even one used on skin
- •
- to make a child sleepy
Ask a doctor before use if you have
- •
- glaucoma
- •
- trouble urinating due to an enlarged prostate gland
- •
- a breathing problem such as emphysema or chronic bronchitis
Directions
- •
- take every 4 to 6 hours, not more than 6 doses in 24 hours
|
adults and children 12 years of age and over |
1 or 2 tablets |
|
children 6 to under 12 years of age |
1 tablet |
|
children 4 to under 6 years of age |
do not use unless directed by a doctor |
|
children under 4 years of age |
do not use |
Inactive ingredients
carnauba wax, crospovidone, D&C red no. 27 aluminum lake, dibasic calcium phosphate, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, starch, stearic acid, titanium dioxide
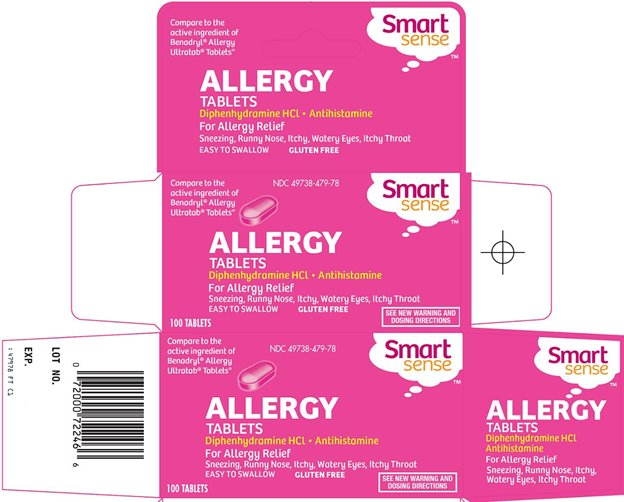
Principal Display Panel
Compare to the active ingredient of Benadryl® Allergy Ultratab® Tablets
ALLERGY TABLETS
Diphenhydramine HCl - Antihistamine
For Allergy Relief
Sneezing, Runny Nose, Itchy, Watery Eyes, Itchy Throat
EASY TO SWALLOW
GLUTEN FREE
SEE NEW WARNING AND DOSING DIRECTIONS
Allergy Tablets Carton Image 2
| SMART SENSE ALLERGY
diphenhydramine hydrochloride tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Kmart Corporation (008965873) |